Download the Acumedia Cross Reference Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reverse Blot Hybridization Assay
Detection of Waterborne Pathogens by Polymerase Chain Reaction- Reverse Blot Hybridization Assay Yeonim Choi The Graduate School Yonsei University Department of Biomedical Laboratory Science Detection of Waterborne Pathogens by Polymerase Chain Reaction- Reverse Blot Hybridization Assay A Dissertation Submitted to the Department of Biomedical Laboratory Science and the Graduate School of Yonsei University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Yeonim Choi July 2011 G This certifies that the dissertation of Yeonim Choi is approved. Thesis Supervisor : Hyeyoung Lee Ok Doo Awh : Thesis Committee Member Tae Ue Kim : Thesis Committee Member Jong Bae Kim : Thesis Committee Member Yong Serk Park : Thesis Committee Member The Graduate School Yonsei University July 2011 G G Dedicated to my family and my friends, who have encouraged me. G G CONTENTS LIST OF FIGURES AND TABLES ------------------------------------------------ iv ABBREVIATIONS ------------------------------------------------------------------- ix ABSTRACT IN ENGLISH ----------------------------------------------------------- x I. INTRODUCTION ------------------------------------------------------------- 1 II. MATERIALS AND METHODS -------------------------------------------- 9 1. Development of PCR-REBA targeting waterborne pathogens -------- 9 Bacterial reference strains and cultivation ------------------------------- 9 Genomic DNA extraction -

Culture Media Edition for Industrial Microbiology LABORATORIOS CONDA S.A
2nd Edition for Industrial Microbiology Culture Media LABORATORIOS CONDA S.A. Edited by: Laboratorios Conda S.A. © 2013. Conda S.A. All rights reserved. Printed in Spain C/ La Forja, 9 28850 - Torrejón de Ardoz, Madrid - SPAIN Tel. +34 91 761 02 00 Fax +34 91 656 82 28 C/ Berlín, 63 08029 Barcelona - SPAIN Tel. +34 93 363 72 64 / 65 Fax. +34 93 363 72 61 [email protected] [email protected] www.condalab.com Index 6 Meat & Fish Industry 20 Beer Industry 10 Water & Beverages 21 Waste Water 11 Dairy Products 23 Cosmetic Industry 15 Bakery 24 Pharmaceutical Industry 17 Processed Foods 25 Microbiology Dehydrated Culture Media Guide 19 Wines iv Media for Industrial Microbiology CULTURE MEDIA FOR INDUSTRIAL MICROBIOLOGY | 2ND EDITION Media for Industrial Microbiology 5 Culture Media for Industrial Microbiology Laboratorios CONDA, one of the world USP and AOAC standards. Strict quality leaders in the design and manufacturing of control procedures are adopted prior to, high quality culture media, currently offers during and after the manufacturing process more than 400 different products, among to ensure quality products and batch-to-batch which you will find chromogenic media, ISO- consistency. We also exert tight control over formulated media and custom-made media selection and treatment of all raw materials for many different industrial applications. and components (peptones, carbohydrates, minerals, chemicals, agar and other additives) From hygiene control, through food used in the manufacturing process. Physical- and beverage poisoning prevention, to chemical characteristics are tested, and microbiologial examination of cosmetic and media also undergo additional microbiological pharmaceutical products, CONDA supplies a tests that guarantee growth, differentiation, wide variety of different media for each field biochemical performance, recovery of small so that customers can find the most suitable inocula, selectivity, etc. -

Pocket Guide to Clinical Microbiology
4TH EDITION Pocket Guide to Clinical Microbiology Christopher D. Doern 4TH EDITION POCKET GUIDE TO Clinical Microbiology 4TH EDITION POCKET GUIDE TO Clinical Microbiology Christopher D. Doern, PhD, D(ABMM) Assistant Professor, Pathology Director of Clinical Microbiology Virginia Commonwealth University Health System Medical College of Virginia Campus Washington, DC Copyright © 2018 Amer i can Society for Microbiology. All rights re served. No part of this publi ca tion may be re pro duced or trans mit ted in whole or in part or re used in any form or by any means, elec tronic or me chan i cal, in clud ing pho to copy ing and re cord ing, or by any in for ma tion stor age and re trieval sys tem, with out per mis sion in writ ing from the pub lish er. Disclaimer: To the best of the pub lish er’s knowl edge, this pub li ca tion pro vi des in for ma tion con cern ing the sub ject mat ter cov ered that is ac cu rate as of the date of pub li ca tion. The pub lisher is not pro vid ing le gal, med i cal, or other pro fes sional ser vices. Any ref er ence herein to any spe cific com mer cial prod ucts, pro ce dures, or ser vices by trade name, trade mark, man u fac turer, or oth er wise does not con sti tute or im ply en dorse ment, rec om men da tion, or fa vored sta tus by the Ameri can Society for Microbiology (ASM). -

PDF, Effect of Differences in Salt Concentration on the Quality Of
IOP Conference Series: Earth and Environmental Science PAPER • OPEN ACCESS Effect of Differences in Salt Concentration on the Quality of Rebon Shrimp Paste (Acetes Sp) in Tegal District To cite this article: S Mulyani et al 2021 IOP Conf. Ser.: Earth Environ. Sci. 755 012051 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.33.19 on 26/09/2021 at 20:52 ACHOST 2020 IOP Publishing IOP Conf. Series: Earth and Environmental Science 755 (2021) 012051 doi:10.1088/1755-1315/755/1/012051 Effect of Differences in Salt Concentration on the Quality of Rebon Shrimp Paste (Acetes Sp) in Tegal District S Mulyani 1*, P M Vestiyati 1, Kusnandar 1, H K Alamsyah 1, and S W Simanjuntak 1 1Faculty of Fisheries and Marine Science, Pancasakti University of Tegal, Indonesia *[email protected] Abstract. Rebon Shrimp Paste (RSP) in Indonesia uses different percentages of salt addition, ranging from 2 to 20% or not at all. This study aims to determine the influence of different salt concentration (5%, 10%, 15% and without salt) on the quality of RSP organoleptic, microbiological and chemical. This research was conducted in Munjung Agung, Tegal and Cirebon Fisheries Product Quality Testing and Application Laboratory. The results showed that the addition of different salt concentration (5%, 10%,15% and without salt) affected the quality of organoleptics, microbiology, and chemistry. Organoleptic quality with salt concentration of 5% and 10% favored panelists with an average value of 6.8 (not yet meeting Indonesian National Standards). The highest water content value is found in RSP that are not added salt (40,19%-43,22%) and lowest at 15% salt concentration (31,12%-34,82%) in accordance with the SNI. -

Antimicrobial Resistance EMERGING INFECTIOUS DISEASES Pages 681-814 Peer-Reviewed Journal Tracking and Analyzing Disease Trends Pages 681–814
Vol 13, No 5, May 2007 Vol ® May 2007 Antimicrobial Resistance EMERGING INFECTIOUS DISEASES Pages 681-814 Pages Peer-Reviewed Journal Tracking and Analyzing Disease Trends pages 681–814 EDITOR-IN-CHIEF D. Peter Drotman EDITORIAL STAFF EDITORIAL BOARD Managing Senior Editor Dennis Alexander, Addlestone Surrey, United Kingdom Polyxeni Potter, Atlanta, Georgia, USA Barry J. Beaty, Ft. Collins, Colorado, USA Associate Editors Martin J. Blaser, New York, New York, USA Paul Arguin, Atlanta, Georgia, USA David Brandling-Bennet, Washington, D.C., USA Charles Ben Beard, Ft. Collins, Colorado, USA Donald S. Burke, Baltimore, Maryland, USA David Bell, Atlanta, Georgia, USA Arturo Casadevall, New York, New York, USA Jay C. Butler, Anchorage, Alaska, USA Kenneth C. Castro, Atlanta, Georgia, USA Charles H. Calisher, Ft. Collins, Colorado, USA Thomas Cleary, Houston, Texas, USA Stephanie James, Bethesda, Maryland, USA Anne DeGroot, Providence, Rhode Island, USA Brian W.J. Mahy, Atlanta, Georgia, USA Vincent Deubel, Shanghai, China Paul V. Effler, Honolulu, Hawaii, USA Nina Marano, Atlanta, Georgia, USA Ed Eitzen, Washington, D.C., USA Martin I. Meltzer, Atlanta, Georgia, USA Duane J. Gubler, Honolulu, Hawaii, USA David Morens, Bethesda, Maryland, USA Richard L. Guerrant, Charlottesville, Virginia, USA J. Glenn Morris, Baltimore, Maryland, USA Scott Halstead, Arlington, Virginia, USA Marguerite Pappaioanou, St. Paul, Minnesota, USA David L. Heymann, Geneva, Switzerland Tanja Popovic, Atlanta, Georgia, USA Daniel B. Jernigan, Atlanta, Georgia, USA Patricia M. Quinlisk, Des Moines, Iowa, USA Charles King, Cleveland, Ohio, USA Jocelyn A. Rankin, Atlanta, Georgia, USA Keith Klugman, Atlanta, Georgia, USA Didier Raoult, Marseilles, France Takeshi Kurata, Tokyo, Japan Pierre Rollin, Atlanta, Georgia, USA S.K. -

Food Microbiology
Food Microbiology Food Water Dairy Beverage Online Ordering Available Food, Water, Dairy, & Beverage Microbiology Table of Contents 1 Environmental Monitoring Contact Plates 3 Petri Plates 3 Culture Media for Air Sampling 4 Environmental Sampling Boot Swabs 6 Environmental Testing Swabs 8 Surface Sanitizers 8 Hand Sanitation 9 Sample Preparation - Dilution Vials 10 Compact Dry™ 12 HardyCHROM™ Chromogenic Culture Media 15 Prepared Media 24 Agar Plates for Membrane Filtration 26 CRITERION™ Dehydrated Culture Media 28 Pathogen Detection Environmental With Monitoring Contact Plates Baird Parker Agar Friction Lid For the selective isolation and enumeration of coagulase-positive staphylococci (Staphylococcus aureus) on environmental surfaces. HardyCHROM™ ECC 15x60mm contact plate, A chromogenic medium for the detection, 10/pk ................................................................................ 89407-364 differentiation, and enumeration of Escherichia coli and other coliforms from environmental surfaces (E. coli D/E Neutralizing Agar turns blue, coliforms turn red). For the enumeration of environmental organisms. 15x60mm plate contact plate, The media is able to neutralize most antiseptics 10/pk ................................................................................ 89407-354 and disinfectants that may inhibit the growth of environmental organisms. Malt Extract 15x60mm contact plate, Malt Extract is recommended for the cultivation and 10/pk ................................................................................89407-482 -

APPENDIX a Media and Reagents
APPENDIX A Media and Reagents Pauline K. w. Yu, M.S. The use of appropriate and dependable media is integral to the isolation and identification of microorganisms. Unfortunately, comparative data docu menting the relative efficacy or value of media designed for similar purposes are often lacking. Moreover, one cannot presume identity in composition of a given generic product which is manufactured by several companies because each may supplement the generic products with components, often of a proprietary nature and not specified in the product's labeling. Finally, the actual production of similar products may vary among manufacturers to a sufficient extent to affect their performance. For all of these reasons, therefore, product selection for the laboratory should not be strictly based on cost considerations and should certainly not be based on promotional materials. Evaluations that have been published in the scientific literature should be consulted when available. Alternatively, the prospective buyer should consult a recognized authority in the field. It is seldom necessary for the laboratory to prepare media using basic components since these are usually available combined in dehydrated form from commercial sources; however, knowledge of a medium's basic compo nents is helpful in understanding how the medium works and what might be wrong when it does not work. Hence, the components have been listed for each medium included in this chapter. All dehydrated media must be prepared exactly according to the manu facturers' directions. Any deviation from these directions may adversely affect or significantly alter a medium's performance. Containers of media should be dated on receipt and when opened, and the media should never be used beyond expiration dates specified by the manufacturers or recom mended by quality control programs. -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Florula of Larval and Imaginal Phases of the Volfartova Fly (Wohlfarthia magnifica) In the Conditions of the Steppe Zone of The Pavlodar Region. A A Bitkeyeva1* and L T Bulekbayeva2. 1Senior teacher, Master of Ecology, Pavlodar State University named after S. Toraygyrov, The Republic of Kazakhstan. 2Associate professor, Candidate of Biological Sciences, Pavlodar State Pedagogical Institute, Republic of Kazakhstan. ABSTRACT Groups of bacteria were found during research in a steppe zone of the Pavlodar region, belonging to 3 families: Baccilaceae, Micrococcaceae, Enterobacteriacea. 13 species of pathogenic and opportunistic bacteria are obtained and identified, which cause diseases. Reception of agents from flies of Wohlfartia magnifica family in region farms forces to pay attention to quite real possibility and contagion of various infections. It creates the menacing epidemiological and epizootiology situation on the adjacent to farms of populated places, as flies with excrements can infect forages and migrate on considerable distances. Keywords: bacteria, diseases, infections, larvaes, microorganisms, flies, sheep, pathogenic microorganisms, carriers. *Corresponding author July– August 2015 RJPBCS 6(4) Page No. 2069 ISSN: 0975-8585 INTRODUCTION Flies are known as carriers of causative agents of dangerous infectious and invasive diseases. Therefore, in the populated places and on the pastures, studying of microbal and helminthosis impurity of flies represents scientific and practical interest. Epidemiological value of flies was opened by E.N. Pavlovskiy and V.P. Derbeneva-Ukhova, they participate in distribution about 70 pathogenic microflora, and including agents of a tularemia, anthrax, diphtheria, cholera, plague, a crab hand, etc. [2; 8; 12]. -

Clinical Microbiology 12Th Edition
Volume 1 Manual of Clinical Microbiology 12th Edition Downloaded from www.asmscience.org by IP: 94.66.220.5 MCM12_FM.indd 1 On: Thu, 18 Apr 2019 08:17:55 2/12/19 6:48 PM Volume 1 Manual of Clinical Microbiology 12th Edition EDITORS-IN-CHIEF Karen C. Carroll Michael A. Pfaller Division of Medical Microbiology, Departments of Pathology and Epidemiology Department of Pathology, The Johns Hopkins (Emeritus), University of Iowa, University School of Medicine, Iowa City, and JMI Laboratories, Baltimore, Maryland North Liberty, Iowa VOLUME EDITORS Marie Louise Landry Robin Patel Laboratory Medicine and Internal Medicine, Infectious Diseases Research Laboratory, Yale University, New Haven, Connecticut Mayo Clinic, Rochester, Minnesota Alexander J. McAdam Sandra S. Richter Department of Laboratory Medicine, Boston Department of Laboratory Medicine, Children’s Hospital, Boston, Massachusetts Cleveland Clinic, Cleveland, Ohio David W. Warnock Atlanta, Georgia Washington, DC Downloaded from www.asmscience.org by IP: 94.66.220.5 MCM12_FM.indd 2 On: Thu, 18 Apr 2019 08:17:55 2/12/19 6:48 PM Volume 1 Manual of Clinical Microbiology 12th Edition EDITORS-IN-CHIEF Karen C. Carroll Michael A. Pfaller Division of Medical Microbiology, Departments of Pathology and Epidemiology Department of Pathology, The Johns Hopkins (Emeritus), University of Iowa, University School of Medicine, Iowa City, and JMI Laboratories, Baltimore, Maryland North Liberty, Iowa VOLUME EDITORS Marie Louise Landry Robin Patel Laboratory Medicine and Internal Medicine, Infectious Diseases Research Laboratory, Yale University, New Haven, Connecticut Mayo Clinic, Rochester, Minnesota Alexander J. McAdam Sandra S. Richter Department of Laboratory Medicine, Boston Department of Laboratory Medicine, Children’s Hospital, Boston, Massachusetts Cleveland Clinic, Cleveland, Ohio David W. -

BD Industry Catalog
PRODUCT CATALOG INDUSTRIAL MICROBIOLOGY BD Diagnostics Diagnostic Systems Table of Contents Table of Contents 1. Dehydrated Culture Media and Ingredients 5. Stains & Reagents 1.1 Dehydrated Culture Media and Ingredients .................................................................3 5.1 Gram Stains (Kits) ......................................................................................................75 1.1.1 Dehydrated Culture Media ......................................................................................... 3 5.2 Stains and Indicators ..................................................................................................75 5 1.1.2 Additives ...................................................................................................................31 5.3. Reagents and Enzymes ..............................................................................................75 1.2 Media and Ingredients ...............................................................................................34 1 6. Identification and Quality Control Products 1.2.1 Enrichments and Enzymes .........................................................................................34 6.1 BBL™ Crystal™ Identification Systems ..........................................................................79 1.2.2 Meat Peptones and Media ........................................................................................35 6.2 BBL™ Dryslide™ ..........................................................................................................80 -

Bismuth Sulfite Agar
Bismuth Sulfite Agar Intended Use mended for use in testing clinical specimens.15,16 In addition, Bismuth Sulfite Agar is a highly selective medium used for Bismuth Sulfite Agar is valuable when investigating outbreaks of isolating Salmonella spp., particularly Salmonella Typhi, from Salmonella spp., especially S. Typhi.17-19 food and clinical specimens. Bismuth Sulfite Agar is used for the isolation of S. Typhi and other Salmonella from food, feces, urine, sewage and other Summary and Explanation infectious materials. The typhoid organism grows luxuriantly Salmonellosis continues to be an important public health on the medium, forming characteristic black colonies, while problem worldwide, despite efforts to control the prevalence of gram-positive bacteria and members of the coliform group Salmonella in domesticated animals. Infection with nontyphi are inhibited. This inhibitory action of Bismuth Sulfite Agar Salmonella often causes mild, self-limiting illness.1 Typhoid toward gram-positive and coliform organisms permits the use fever, caused by S. Typhi, is characterized by fever, headache, of a much larger inoculum than possible with other media diarrhea and abdominal pain, and can produce fatal respi- employed for similar purposes in the past. The use of larger ratory, hepatic, splenic and/or neurological damage. These inocula greatly increases the possibility of recovering the illnesses result from consumption of raw, undercooked or pathogens, especially when they are present in relatively small improperly processed foods contaminated with Salmonella. numbers. Small numbers of organisms may be encountered in Many cases of Salmonella-related gastroenteritis are due to the early course of the disease or in the checking of carriers improper handling of poultry products. -
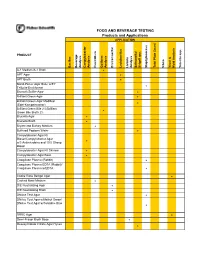
DONE Food and Beverage Testing
FOOD AND BEVERAGE TESTING Products and Applications APPLICATION PRODUCT Bacillus Beverage Analysis Campylobacter Analysis Clostridium Coliform Analysis Environmental Lactobacillus Listeria Analysis Salmonella/ Shigell spp. Staphylococcus Total Plate Count Vibrio Yeast & Mold Analysis Yersinia spp. A-1 Medium/A-1 Broth ! APT Agar ! APT Broth ! Baird-Parker Agar Base w/EY Tellurite Enrichment ! Bismuth Sulfite Agar ! Brilliant Green Agar ! Brilliant Green Agar Modified (Edel-Kampelmacher) ! Brilliant Green Bile 2%/Brilliant Green Bile Broth 2% ! Brucella Agar ! Brucella Broth ! Bryant and Burkey Medium ! Buffered Peptone Water ! Campylobacter Agar Kit Blaser/Campylobacter Agar w/5 Antimicrobics and 10% Sheep ! Blood Campylobacter Agar Kit Skirrow ! Campylobacter Agar Base ! Coagulase Plasma (Rabbit) ! Coagulase Plasma EDTA (Rabbit)/ Coagulase Plasma w/EDTA ! Cooke Rose Bengal Agar ! Cooked Meat Medium ! D/E Neutralizing Agar ! D/E Neutralizing Broth ! DNAse Test Agar ! DNAse Test Agar w/Methyl Green/ DNAse Test Agar w/Toluidine Blue ! DRBC Agar ! Demi-Fraser Broth Base ! Desoxycholate Citrate Agar Hynes ! FOOD AND BEVERAGE TESTING Products and Applications APPLICATION PRODUCT Bacillus Beverage Analysis Campylobacter Analysis Clostridium Coliform Analysis Environmental Lactobacillus Listeria Analysis Salmonella/ Shigell spp. Staphylococcus Total Plate Count Vibrio Yeast & Mold Analysis Yersinia spp. Differential Reinforced Clostridial Agar ! EC Medium/EC Broth ! EC Medium with MUG/EC Broth w/MUG ! Elliker Broth ! m Endo Agar LES ! m Endo