Cell Count and Size in Relation to Fruit Size Among Strawberry Cultivars Guiwen W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fruits: Kinds and Terms
FRUITS: KINDS AND TERMS THE IMPORTANT PART OF THE LIFE CYCLE OFTEN IGNORED Technically, fruits are the mature ovaries of plants that contain ripe seeds ready for dispersal • Of the many kinds of fruits, there are three basic categories: • Dehiscent fruits that split open to shed their seeds, • Indehiscent dry fruits that retain their seeds and are often dispersed as though they were the seed, and • Indehiscent fleshy fruits that turn color and entice animals to eat them, meanwhile allowing the undigested seeds to pass from the animal’s gut We’ll start with dehiscent fruits. The most basic kind, the follicle, contains a single chamber and opens by one lengthwise slit. The columbine seed pods, three per flower, are follicles A mature columbine follicle Milkweed seed pods are also large follicles. Here the follicle hasn’t yet opened. Here is the milkweed follicle opened The legume is a similar seed pod except it opens by two longitudinal slits, one on either side of the fruit. Here you see seeds displayed from a typical legume. Legumes are only found in the pea family Fabaceae. On this fairy duster legume, you can see the two borders that will later split open. Redbud legumes are colorful before they dry and open Lupine legumes twist as they open, projecting the seeds away from the parent The bur clover modifies its legumes by coiling them and providing them with hooked barbs, only opening later as they dry out. The rattlepods or astragaluses modify their legumes by inflating them for wind dispersal, later opening to shed their seeds. -

Strawberry Plant Structure and Growth Habit E
Strawberry Plant Structure and Growth Habit E. Barclay Poling Professor Emeritus, NC State University Campus Box 7609, Raleigh NC 27695-7609 Introduction The strawberry plant has a short thickened stem (called a “crown”) which has a growing point at the upper end and which forms roots at its base (Fig. 1). New leaves and flower clusters emerge from “fleshy buds” in the crown in the early spring. From a cultural viewpoint, it is desirable in our region to have the formation of 1-2 “side stems” called branch crowns form during the late fall (Fig. 2). Each branch crown will add to the yield of the main crown by producing its own “flower cluster” or what is technically called an inflorescence. Branch crowns and main crowns are structurally identical, and an inflorescence develops at the terminal growing point of each crown (Fig. 3). Crown growth and development occur when temperatures are above 50o F (mainly in the month of October). Average daily temperatures in November below this temperature will slow branch crown formation and floral development. Row covers may be a good option in November for Camarosa to help stimulate further reproductive development. A well-balanced Camarosa strawberry plant will form 3-5 branch crowns by the time fruiting season begins in the spring. There is excellent potential for a 2 + lb crop per plant (> 15 tons per acre) when you can see the formation of 1-2 side crowns in addition to the main crown (center) in late fall/early winter (Fig. 7). In Chandler and Camarosa it is critical not to plant too early in the fall and run the risk of having too many crowns form (try to avoid the development of more than 6 crowns per plant). -

EXTENSION EC1257 Garden Terms: Reproductive Plant Morphology — Black/PMS 186 Seeds, Flowers, and Fruitsextension
4 color EXTENSION EC1257 Garden Terms: Reproductive Plant Morphology — Black/PMS 186 Seeds, Flowers, and FruitsEXTENSION Anne Streich, Horticulture Educator Seeds Seed Formation Seeds are a plant reproductive structure, containing a Pollination is the transfer of pollen from an anther to a fertilized embryo in an arrestedBlack state of development, stigma. This may occur by wind or by pollinators. surrounded by a hard outer covering. They vary greatly Cross pollinated plants are fertilized with pollen in color, shape, size, and texture (Figure 1). Seeds are EXTENSION from other plants. dispersed by a variety of methods including animals, wind, and natural characteristics (puffball of dandelion, Self-pollinated plants are fertilized with pollen wings of maples, etc.). from their own fl owers. Fertilization is the union of the (male) sperm nucleus from the pollen grain and the (female) egg nucleus found in the ovary. If fertilization is successful, the ovule will develop into a seed and the ovary will develop into a fruit. Seed Characteristics Seed coats are the hard outer covering of seeds. They protect seed from diseases, insects and unfavorable environmental conditions. Water must be allowed through the seed coat for germination to occur. Endosperm is a food storage tissue found in seeds. It can be made up of proteins, carbohydrates, or fats. Embryos are immature plants in an arrested state of development. They will begin growth when Figure 1. A seed is a small embryonic plant enclosed in a environmental conditions are favorable. covering called the seed coat. Seeds vary in color, shape, size, and texture. Germination is the process in which seeds begin to grow. -

KEY to FRUIT TYPES 1A. Fruit Derived from Several Ovaries of One Or More Flowers 2A. Fruit Arising from the Several Ovaries of A
KEY to FRUIT TYPES 1a. Fruit derived from several ovaries of one or more flowers 2a. Fruit arising from the several ovaries of as many flowers (examples: pineapple, mulberry) MULTIPLE FRUIT 2b. Fruit arising from the coalescence of several ripened ovaries of one flower (example: raspberry, blackberry) AGGREGATE FRUIT 1b. Fruit derived from a single ovary (simple or compound) 3a. Fruit fleshy or juicy when ripe 4a. Ovary wall of fruit (or pericarp) entirely or in part fleshy 5a. Fruit indehiscent 6a. Ovary wall entirely fleshy (examples: tomato, cranberry, grape, currant, banana, melon [pepo], and citrus fruit [hesperidium]) BERRY 6b. Ovary wall of three distinct layers, the inner one bony (endocarp), the middle fleshy (mesocarp), and the outer "skin- like" (exocarp) (examples: peach, plum, cherry) DRUPE 5b. Fruit dehiscent 7a. Fruit derived from one carpel FOLLICLE 7b. Fruit derived from a compound gynoecium CAPSULE 4b. Ovary wall (e.g., the outer layer of an apple 'core') of fruit papery, surrounded by a fleshy material that represents the coalescent parts of the stamens, petals, sepals, and (some believe) receptacle (examples: apple, pear, quince) POME 3b. Fruit typically dry and usually hardened when ripe 8a. Fruit indehiscent (does not open or dehisce when mature), generally with one seed 9a. Ovary wall of varying thickness, usually not bony 10a. Fruit not winged (examples: buttercup, 'seeds' of strawberry, sunflower family, sedges, grasses [ovary wall adherent to and surrounding seed, may be called caryopsis or grain]) ACHENE 10b. Fruit winged (examples: elm, tulip tree) SAMARA 9b. Ovary wall hardened and bony 11a. Fruit usually > 5mm long (examples: oak, chestnut, hazelnut) NUT 11b. -

Flowering Plant Families of Northwestern California: a Tabular Comparison
CORE Metadata, citation and similar papers at core.ac.uk Provided by Digital Commons@Humboldt State University (HSU) Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 12-2019 Flowering Plant Families of Northwestern California: A Tabular Comparison James P. Smith Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P., "Flowering Plant Families of Northwestern California: A Tabular Comparison" (2019). Botanical Studies. 95. https://digitalcommons.humboldt.edu/botany_jps/95 This Flora of Northwest California-Regional is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. FLOWERING PLANT FAMILIES OF NORTHWESTERN CALIFORNIA: A TABULAR COMPARISON James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University December 2019 Scientific Name Habit Leaves Sexuality • Floral Formula Common Name Fruit Type • Comments Aceraceae TSV SC:O U-m [P] • K 4-5 C 4-5 A 4-10 G (2) Maple Paired samaras • leaves often palmately lobed Acoraceae H S:A U-m • P 3+3 A 6 or G (3) Sweet Flag Berry • aquatic; aromatic rhizomes Aizoaceae HS S:AO B • P [3] 5 [8] A 0-4 Gsi (2-5-4) Ice Plant -
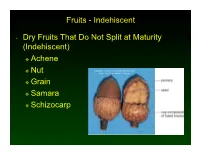
Indehiscent • Dry Fruits That Do Not Split at Maturity
Fruits - Indehiscent • Dry Fruits That Do Not Split at Maturity (Indehiscent) Achene Nut Copyright © McGraw-Hill Companies Permission Required for Reproduction or Display Grain Samara Schizocarp Fruits - Indehiscent • Achene- a single-seeded fruit in which the seed is attached to the pericarp only at its base • The pericarp, the husk, is easily separated from the seed. • Ex. Sunflower, dandelion Fruits - Indehiscent • Nut- achene variation- one seeded, dry fruit with a hard, thick pericarp; develops with a cup or cluster of bracts at base • Ex- acorn, chestnut, hazelnut Fruits - Indehiscent • Grain (caryopsis)- a dry fruit in which the pericarp is tightly fused to the seed • Ex- corn, rice, wheat Fruits - Indehiscent • Samara- a dry fruit whose pericarp extends around the seed in the form of a wing • Ex. Maple, ash Fruits - Indehiscent • Schizocarp- a twin fruit that separates at maturity into two one-seeded fruitlets • Ex- parsley, carrot, dill Fruits • Aggregate Fruits- derived from a single flower with several to many pistils • Individual pistils mature as a clustered unit on a single receptacle • Ex- raspberries, strawberries Fruit • Multiple Fruit- derived from several to many individual flowers in a single inflorescence • Ex. Pineapple, fig, Osage orange, mulberries Fruit and Seed Dispersal • Wind Dispersal Small and Lightweight seeds. May have attachments like wings or hairs to help give them lift. Example- maple, ash, dandelion • Animal Dispersal Seeds can pass through an animal’s digestive tract. Some fruits and seeds have spines or thorns that catch in fur or feathers. Oils attract ants. Fruit and Seed Dispersal • Water Dispersal Some fruits contain trapped air. -

Lecture 27-28. Fruits Topics Formation of Fruits Basic Fruit Types Fruit Types
Lecture 27-28. Fruits Topics • Formation of fruits • Basic Fruit Types Formation of fruits Basic Fruit Types • The two principal Fruit Types are Fleshy & Dry – Caution: A Legume is a dry fruit. We eat unripe legumes like Snow Peas and Green Beans. We might classify the latter as fleshy fruits but they are dry at maturity!! • Dry Fruits are either Dehiscent or Indehiscent. – Dehiscent Fruits open at maturity while indehiscent Fruits do not! • Fruits may be Simple or Accessory. – Simple Fruits are Mature Carpels. – Accessory Fruits include the Carpels & other tissues. • The latter may be other Floral Organs or the Receptacle. • Aggregate vs multiple fruits – Aggregate Fruits contain Many Simple Carpels from ONE FLOWER – Multiple Fruits contain the Fruits of MANY FLOWERS. Fruit Types 1 Fruit Types: simple fruits Simple Fruits • Simple Fruits – Simple fruits are derived from • Fleshy single or several united carpels. - Drupe: peach – Legumes are fruits that split along two sides when mature. - Berry: grape • Dehiscent - Split open - Pome: apple • Indehiscent - Fail to split open •Dry – Dehiscent • Follicle: peony • Legume: bean • Capsule: poppy – Indehiscent • Achene: sunflower • Nut: hazelnut • Grain: rice Simple Fruits – Dispersal • Many seeds are dispersed by wind. – Woolly hairs, plumes, wings – Fleshy fruits - Attract animals • Berry. Fleshy fruit, with succulent and provide them with food. • Peaches, cherries, tomatoes pericarp, as in Vitis. – Accessory fruit - Bulk of fruit is not from ovary, but from • Drupe. A fleshy fruit with a stony receptacle. endocarp, as in Prunus. • Apples • Drupelet. A small drupe, as in Rubus. Berry – from compound ovary with many seeds Drupe – from simple ovary with one seed and soft “skin” • have a fleshy or leathery Grapes (Vitis) • like Berries but they Exocarp, Mesocarp and have Stony Endocarps Endocarp. -

Achene Morphology of British and Irish Mayweeds and Chamomiles: Implications for Taxonomy and Identification
British & Irish Botany 1(2): 128-166, 2019 Achene morphology of British and Irish mayweeds and chamomiles: implications for taxonomy and identification Chris A. Skilbeck*, Iris Lynch, Maggie Ellenby, Mark A. Spencer Department of Life Sciences, Natural History Museum, London, SW7. *Corresponding author: Chris A. Skilbeck, email: [email protected] This pdf constitutes the Version of Record published on 21st May 2019 Abstract Whilst determining the mayweed and chamomile specimens in the Natural History Museum’s British & Irish Herbarium we decided to produce a photographic guide and key to assist in the determination of these plants using achene morphology. Because of our observations, and in line with North American treatments, we propose recognising Matricaria discoidea subsp. discoidea and M. discoidea subsp. occidentalis as separate species. We also discuss possible introgression from Tripleurospermum maritimum subsp. phaeocephalum in northern parts of Birtain and Ireland, the implications for the taxonomy of Anthemis/Cota and Chamaemelum and the usefulness of achene glands in determination of specimens. Finally we consider the problems that may arise when using immature achenes and herbarium specimens for determination and we discuss the usefulness of being aware of changes in colour and form that occur during development, particularly as many collected specimens lacked ripe capitula. Keywords: Anthemis; Cota; Chamaemelum; Matricaria; Tripleurospermum; Introduction Mayweeds and chamomiles can be a difficult group to determine to species and subspecies level and on a global scale their taxonomy is still challenging (Oberprieler, 1998, 2002; Lo Presti et al., 2010). Although there are differences in habit and leaf morphology, there is also tremendous variation within each species. -

Laboratory 2: Reproductive Morphology
IB 168 (Plant Systematics) Laboratory 2: Reproductive Morphology A Review of the Plant Life Cycle All plants have a very characteristic life cycle composed of two distinct phases or generations: a haploid (1N) gametophyte generation and a diploid (2N) sporophyte generation. The gametophyte generation produces gametes (by mitosis) which fuse in the process of fertilization to produce a diploid sporophyte. The sporophyte, in turn, produces haploid spores (by meiosis) which gives rise to new gametophytes. Because the two generations alternate with one another, this kind of life cycle is often referred to as alternation of generations (see FIGURE 1). Some plants are homosporous, in that they produce only one type of spore, which gives rise to a bisexual gametophyte upon germination. Most plants, however, produce two morphologically different types of spores and are thus heterosporous. The larger of these spores is termed the megaspore and the smaller one the microspore. Upon germination megaspores will give rise to female gametophytes (megagametophytes) and microspores will germinate to form male gametophytes (microgametophytes). One important consequence of heterospory is that gametophytes are now rendered unisexual. Heterospory has evolved at least four times in the history of plants, yet, although it is regarded as a key evolutionary step, the advantages (if any) of heterospory have proven difficult to assess. Sporophyte DIPLOID (2N) Fertilization Meiosis HAPLOID (1N) gametes spores Gametophyte FIGURE 1: Generalized plant life cycle. Note that the spores are haploid because they are the products of meiosis. These spores germinate to form a haploid gametophyte. The gametophyte then produces gametes (eggs or sperm) by mitosis. -
Overcoming Strawberry Achene Dormancy for Improved Seedling
Volumen 32, Nº 4. Páginas 57-62 IDESIA (Chile) Septiembre-Noviembre, 2014 Overcoming strawberry achene dormancy for improved seedling production in breeding programs Superación de la latencia de los aquenios de la fresa para mejorar la producción de plántulas en programas de mejoramiento Alexandre Gonçalves Galvão1*, Luciane Vilela Resende1, Renato Mendes Guimaraes1, Andrew Kim Lopes Ferraz1, Rafael Gustavo Ferreira Morales2, Josué Clock Marodin3, Hugo Cesar Rodrigues Moreira Catão1 ABSTRACT Strawberry achenes present tegument dormancy. This fact, together with the low efficiency of artificial pollination requires a larger number of crossings to ensure the minimum number of achenes used in seedling production in breeding programs. The aim of this study was to test chemical solutions to overcome dormancy in strawberry achenes in order to increase the germination and Germination Speed Index (GSI). The study consisted of two experiments. 1) immersion in H2SO4 (98%), HCl (37%) and NaClO (2%) for 0 (control), 10, 20, 35 and 50 min were evaluated. 2) achenes were immersed in H2SO4 (98%) for 0 (control), 20, 25, 30, 35 and 40 min. A completely randomized design with four replications was used. Germination and GSI were assessed daily for 15 days. Scarification with H2SO4 (98%) for 40 min increased strawberry achene germination to 80% and can be used as a technique to overcome dormancy. Scarification with HCl and NaClO increases germination and the GSI, however, other concentrations and immersion times of those methods should be studied. Key words: fragaria x ananassa Duch, strawberry breeding, tegumentary dormancy, acid scarification RESUMEN Los aquenios de la fresa presentan latencia del tegumento. -
Bio 320 - Ethnobotany April 16, 2015 Basic Plant Identification
1 Bio 320 - Ethnobotany April 16, 2015 Basic Plant Identification I. Terminology and vocabulary It is important that we develop a good working knowledge of botanical terminology and vocabulary - thus we will spend today working on developing that knowledge. This handout lists many terms that are necessary for identifying plants via a key. Don’t worry about memorizing them all, but you should feel comfortable with them. I am also giving you a copy of a key to families of plants native to Illinois. You should use that key to work some through the demonstration plants. Also be sure to examine the herbarium sheets for examples of interesting local plants. Handle the herbarium sheets very carefully. They are fragile. A. Habit - extremely important in identifying plants grossly - trees, shrubs, herbs woody - trees - large, 1 main stem - shrubs - smaller, several main stems herbs - no well developed woody layer suffrutescent - only slightly woody, can be large - often upper parts of stems are herbaceous suffruticose - diminutive shrub - very woody habit - stems acaulescent - no stem - a dandelion caulescent - have stem cespitose - in little tufts or dense clumps - used for small plants growing in turf B. Plant parts Vegetative parts - roots, stems, leaves Reproductive parts - flowers and fruits Roots - often ignored in identifying plants, though can be important taproot - more or less fleshy, goes straight into ground - many dicots fibrous roots - bushy, thin fibers - all monocots Stems can also be important in identification - herbaceous vs. -
Flowering and Fruiting on Command in Berry Crops
Flowering and Fruiting on Command in Berry Crops B.C. Strik Department of Horticulture Oregon State University 4017 ALS, Corvallis, OR 97331 USA Keywords: Fragaria × ananassa, Rubus idaeus, Vaccinium corymbosum, blueberry, blackberry, strawberry, flower bud initiation, photoperiod, off-season production, tunnels, primocane fruiting, day-neutral, everbearer Abstract Many berry crops are managed by altering growth and environment in commercial production systems, to affect time of flowering and fruiting season. The effect of photoperiod, temperature, and production system on flower bud initiation and development is reviewed for strawberry, raspberry, blackberry and blueberry. While “flowering on command” is still a challenge, research and commercial production systems have been successful at scheduling fruit harvest for many of these crops. In strawberry and biennial cropping red raspberry, pre-conditioned plants, grown to maximize flower bud development, are commonly used along with staggered planting dates (often in tunnels) to time fruit harvest date. In primocane-fruiting raspberry, annual production systems with staggered planting dates, often in combination with pruning techniques, are used to target market windows. This system of production shows promise with primocane-fruiting blackberry. In southern highbush blueberry, cultivars are grown in warm regions using evergreening systems, pruning, and fertilization methods, to produce fruit at desired times. With a good understanding of plant physiology and the factors that affect flower bud initiation and development, bud break, and the production of high-quality fruit, it is possible to manipulate many berry crops to have fruit production target desirable market windows. INTRODUCTION There are various ways to control flowering and fruiting season including use of plants with “pre-formed flower buds”, continuous cropping types or cultivars, climate modification (including protected culture), and growing crops at low-latitude or in a milder climate.