HEMP and CBD REGULATORY FACT SHEET
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sunn Hemp Shines in New England
Sunn Hemp Shines in Massachusetts Sam Corcoran & Masoud Hashemi Foreground: Testing out sunn hemp as mulch and fertilizer for garlic, fall 2017; Background: a field of flowering sunn hemp before winterkill, early November. Sunn hemp is a new, summer crop for us in the Northeast. Despite its name, Sunn hemp (Crotalaria juncea) is not related to the industrial hemp you may be familiar with (Cannabis sativa). The Sunn Hemp plant bears only a mild resemblance to Cannabis, and is actually a legume in the same family as peas and beans. As a legume, Sunn Hemp has a relationship with bacteria that convert atmospheric nitrogen into plant-available nitrogen. It is believed that this tropical crop has been grown for hundreds of years, and it remains popular in India, Bangladesh, and Brazil. Sunn Hemp can be used for for- age, fiber, or as a green manure to provide nitrogen to subsequently planted crops. Modern interest in Sunn Hemp in the U.S. surged in Hawaii in the 80’s. Research spread across the southern U.S. in the 90’s through present, with Mid-Atlantic States also taking a recent research inter- est. Within just the past 2-3 years, a few seed companies have start- ed readily supplying Sunn Hemp throughout the U.S. Four years ago, we tried planting this crop at the UMass Research Farm and discovered we can grow Sunn Hemp, too. Despite our cooler climate, the hot summers in Massachusetts are sufficient for this tropical crop; in the 2016 drought, Sunn Hemp remained high performing while other crops suffered. -

Impact of Hemp Legalization on Safety Oversight of Cmv Drivers Office of Drug and Alcohol Policy and Compliance (Odapc)
IMPACT OF HEMP LEGALIZATION ON SAFETY OVERSIGHT OF CMV DRIVERS OFFICE OF DRUG AND ALCOHOL POLICY AND COMPLIANCE (ODAPC) • Author and guardian of 49 CFR Part 40, throughout the Department: • Federal Aviation Administration (FAA) • Federal Motor Carrier Safety Administration (FMCSA) • Federal Railroad Administration (FRA) • Federal Transit Administration (FTA) • Pipeline and Hazardous Materials Safety Administration (PHMSA), as well as for the • US Coast Guard (USCG) • ODAPC provides intermodal coordination and ensures a ONE-DOT approach to regulatory, policy, and compliance matters. 2 DOT DRUG TESTING • Drug tests detect recent drug use - NOT impairment. • No impairment standard for drug levels like alcohol levels. • Windows of detection for each drug varies, but marijuana may be detected for up to 30 days based on the employee’s use and physical build. • DOT tests for: • Marijuana (THC) • Opioids (OPI) • Cocaine (COC) • Codeine • Amphetamines (AMP) • Morphine • Amphetamine • 6-AM (heroin) • Methamphetamine • Hydrocodone • MDMA • Hydromorphone • MDA • Oxycodone • Oxymorphone • Phencyclidine (PCP) 3 HEMP AS DEFINED BY THE 2018 FARM BILL Agricultural Marketing Act of 1946 [amended to include] Subtitle G— Hemp Production SEC. 297A. [7 U.S.C. 1639o] DEFINITIONS. (1) HEMP.—The term ‘‘hemp’’ means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis. cannabinoids = THC (Δ9-THC, Δ8-THC), CBD, CBV, CBN, CBC, CBG, CBL…. 4 HEMP VS MARIJUANA Hemp Marijuana • Cannabis sativa L. species • Cannabis sativa L. -

Sproutman's Hemp Sprouting
Sproutman’s Hemp Sprouting Bag Invented circa 1979 by Steve Meyerowitz, Sproutman® 1. Sterilize your new sprout bag by turning it inside cause problems. 3. Soak ½ cup of out and bathing it in boiling water for only 5 minutes. seed (see chart) in a jar overnight—- 2. Purchase seeds that are specifically adapted for about 8 hours—no more. Use a jar sprouting. Seeds from food store bulk bins typically with 16-32 ounces of pure water. Leave hanging or set in a bowl after dripping stops 4. After the 8 hrs., pour the soaked seeds into the wet, pre-washed sprout bag. Pull the draw string closed. Rinse by dipping the bag into a bowl of water or soaking it in the sink. Soak for at least 1 minute. Then hang it on a hook or knob or lay it in the dish rack or dishwasher rack. 5. Rinse twice per day, about 12 hours apart. Think of feeding them (watering) when you have breakfast and dinner. Just dip and hang! It only takes a minute! You’ve now got the basic steps. Variety #Grow Days Amount Skill Level Spelt 2-3 4-8 oz Easy Hard Wheat 2-3 4-8 oz Easy Kamut 2-3 4-8 oz Easy Soft Wheat 2-3 4-8 oz Easy Green Pea 4-5 4-8 oz Easy Lentil 4-5 4-8 oz Easy Mung 4-5 4-8 oz Easy Hulled Sunflower 2 4-8 oz Easy Radish 5-6 2-3 oz Easy Adzuki 4-5 4-8 oz Medium Broccoli 6 2-3 oz Medium Fenugreek 6 2-3 oz Medium Alfalfa 6-7 2-3 oz Medium Clover 6-7 2-3 oz Medium Chick Pea 4-5 4-8 oz Hard Soybean 4-5 4-8 oz Hard Chia 12 2-3 oz Very Hard About the Chart The sprout bag is very versatile and grows most sprout seeds. -

Hemp Testing Guidelines Issued January 15, 2021
Laboratory Testing Guidelines U.S. Domestic Hemp Production Program Issued January 15, 2021 Purpose: 1. Standard testing procedures are specified for samples taken in accordance with the Sampling Procedures for the USDA Hemp Production Program to measure the total delta-9 tetrahydrocannabinol (THC) concentration levels of samples on a dry weight basis. 2. The results are intended to measure the total THC concentration of composite hemp samples collected from a “lot” of hemp crop acreage designated by a hemp producer and as reported to USDA as required under the USDA Hemp Production Program. The purpose of the measurements is to determine whether the total THC concentration of the tested material is within the acceptable hemp THC level. Scope: 1. Hemp grown under a USDA, State, or Tribal hemp production plan is subject to sampling and compliance testing for THC concentration. Certain producers, including research institutions and facilities growing immature plants may have different testing requirements depending on the applicable State or Tribal plan and regulations. 2. Tests shall measure the total THC concentration in a sample submitted to a laboratory for analysis. The laboratory will perform chemical analysis on the sample using post- decarboxylation or other similarly reliable methods where the total THC concentration level considers the potential to convert delta-9-tetrahydrocannabinolic acid (THCA) into THC. 3. The total delta-9 tetrahydrocannabinol concentration level shall be determined and reported on a dry weight basis. 4. Laboratories shall calculate and include the Measurement of Uncertainty (MU) when they report THC concentration test results. “Measurement of uncertainty” is defined as “the parameter, associated with the result of a measurement, that characterizes the dispersion of the values that could reasonably be attributed to the particular quantity subject to measurement.” USDA does not establish or standardize an upper or lower boundary for general use by laboratories to calculate a measurement of uncertainty. -
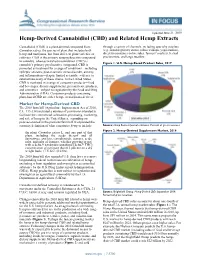
Hemp-Derived Cannabidiol (CBD) and Related Hemp Extracts
Updated June 21, 2019 Hemp-Derived Cannabidiol (CBD) and Related Hemp Extracts Cannabidiol (CBD) is a plant-derived compound from through a variety of channels, including specialty retailers Cannabis sativa, the species of plant that includes both (e.g., natural grocery stores, tobacco shops, yoga studios), hemp and marijuana, but from different plant varieties or direct-to-consumer online sales, farmers’ markets, herbal cultivars. CBD is the primary nonpsychoactive compound practitioners, and large retailers. in cannabis, whereas tetrahydrocannabinol (THC) is cannabis’s primary psychoactive compound. CBD is Figure 1. U.S. Hemp-Based Product Sales, 2017 promoted as treatment for a range of conditions—including epileptic seizures, post-traumatic stress disorder, anxiety, and inflammation—despite limited scientific evidence to substantiate many of these claims. In the United States, CBD is marketed in a range of consumer products—food and beverages, dietary supplements, personal care products, and cosmetics—subject to regulation by the Food and Drug Administration (FDA). Consumer products containing plant-based CBD are either hemp- or marijuana-derived. Market for Hemp-Derived CBD The 2018 farm bill (Agriculture Improvement Act of 2018, P.L. 115-334) included a number of provisions intended to facilitate the commercial cultivation, processing, marketing, and sale of hemp in the United States, expanding on policies enacted in the previous farm bill. It expanded the statutory definition of what constitutes hemp to include: Source: Hemp Business Journal estimates. Percent of gross revenues. Figure 2. Hemp-Derived Supplement Market, 2018 the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol [delta-9 THC] concentration of not more than 0.3 percent on a dry weight basis. -

Autoimmune Protocol Food List
Autoimmune Protocol Food List The Paleo Autoimmune Protocol (AIP) eliminates certain primal foods that can sometimes trigger inflammation in people with autoimmune disease (dairy, eggs, nightshades, nuts and seeds). AIP isn’t necessarily easy – it definitely takes commitment. If you have an autoimmune condition and you want to get on top of it, then you’re going to need to do AIP 100%. We want everyone to be able to enjoy the benefits of our 10 Week Program and reclaim their health, so we have designed recipes to suit those following this protocol. We have also put together this supporting list of foods that are suitable for an Autoimmune Protocol. © PETE EVANS CHEF PTY LTD 2015. ALL RIGHTS RESERVED © PETE EVANS CHEF PTY LTD 2015. ALL RIGHTS RESERVED Vegetables & Fruits Protein Fats Organic is best Naturally pasture fed and sustainably raised EAT EAT EAT Vegetables of all kinds (8 – 14 cups / day) Quality meats (Pastured, grass-fed, organic) Avocados As much variety as much possible Poultry in moderation due to high omega-6 Coconut Colourful vegetables and fruit Beef Fatty fish Cruciferous vegetables Buffalo Pastured, grass-fed animal fats Arugula Chicken Olives Broccoli Duck Brussels sprouts Elk Probiotic foods Cabbage Lamb Kale Pheasant EAT Mustard greens Pork Coconut milk kefir Turnips Rabbit Coconut milk yogurt Watercress Turkey Fermented vegetables or fruit Sea Vegetables Venison Kombucha Excluding algae e.g. Chlorella, Spirulina Wild boar Water kefir Organ meat and offal NOTE: AVOID Aim for 5 times a week, the more the better You can also improve your intake of Nightshades Fish and shellfish (Wild is best) important trace minerals by switching to Himalayan pink salt or “dirty” sea salt. -

A Bottom up Proposal for a Regulated Cannabis Market
A BOTTOM UP PROPOSAL FOR A REGULATED CANNABIS MARKET By Joep Oomen, European Coalition for Just and Effective Drug Policies Wellcome and thanks for the invitation…. Encod is convinced that a Europe with peace, prosperity, personal rights and freedoms, effective governance, policies aimed at solving problems and not at creating them, is a Europe where the drugsmarket has been regulated. The lack of a legal framework with regards to drugs is one of the pillars of a system that essentially denies human beings legal access to products (some of them even natural products) which are benefitial to their health, physical or mental. Among the many disasters that prohibition of cannabis and other drugs has produced, maybe the worst is that it has destroyed this access, and instead established a market that is almost entirely driven by economic reasons, without taking into account the rights of consumers and producers. This lack of framework is deliberately maintained so on the one hand, power structures can continue to feed themselves with money gained from desperacy, while on the other, power structures can continue to justify fargoing control and intrusion in people’s private life. Drug prohibition is serving the needs of the elite, and therefore it will not be the elite that will be able to stop it. If we want to replace this policy with a sound and healthy alternative, we need to build it ourselves, bottom up. Since more than a decade, citizens allover Europe have started to use the loopholes in the absurd drug law to create this alternative. Wellcome to the Cannabis Social Club! Cannabis Social Clubs organise the collective cultivation of an amount of cannabis that is exclusively meant for the private consumption of their members. -

Hemp Recipes
Hemp Recipes © Daphne Lambert Hemp drink There are 2 ways of making this drink either with un-hulled or hulled seeds. You can vary the ratios depending on your taste and texture preference. 100g un-hulled hemp seeds 400ml water blender, sieve, muslin Add the hemp and water to a blender and blend for around a minute or until smooth with no visible husks. Place the muslin cloth into a sieve or colander over a large bowl or jug and pour in the milk. Squeeze the cloth to get out as much of the milk as you can. Add the husk pulp to your compost. If you have a very good high powered blender you may find there is very little husk left after blending and none to remove. It depends how smooth you want the end product. Store in a glass bottle in the fridge for up to 2/3 days 100g hulled hemp seeds 500ml water blender Blend well. Will keep in the fridge for up to 2/3 days Chocolate hemp drink serves 2 75g hulled hemp seeds ½ litre water 2 teaspoons raw cacao powder 1 teaspoon honey Blend well together. Will keep in the fridge for up to 2/3 days Buckwheat & hemp breakfast serves 1 handful of sprouted buckwheat 1 dessertspoon pumpkin seeds 1 dessertspoon sunflower seeds 1 teaspoon honey 120 ml hemp drink seasonal fruit (blackberries, chopped apple, poached rhubarb, strawberries) 1 heaped tablespoon hulled hemp seeds Soak the buckwheat and seeds with the honey in the hemp drink overnight. Serve topped with your chosen fruit and hemp seeds. -

Industrial Hemp (Cannabis Sativa Subsp
molecules Review Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals H. P. Vasantha Rupasinghe 1,* , Amy Davis 1, Shanthanu K. Kumar 2, Beth Murray 1 and Valtcho D. Zheljazkov 3 1 Department of Plant, Food, and Environmental Sciences, Faculty of Agriculture, Dalhousie University, Truro, NS B2N 5E3, Canada; [email protected] (A.D.); [email protected] (B.M.) 2 Section of Horticulture, School of Integrative Plant Science, College of Agriculture and Life Sciences, Cornell University, Ithaca, NY 14850, USA; [email protected] 3 Department of Crop and Soil Science, 431A Crop Science Building, 3050 SW Campus Way, Oregon State University, Corvallis, OR 97331, USA; [email protected] * Correspondence: [email protected] Academic Editor: Severina Pacifico Received: 5 August 2020; Accepted: 2 September 2020; Published: 7 September 2020 Abstract: Industrial hemp (Cannabis sativa L., Cannabaceae) is an ancient cultivated plant originating from Central Asia and historically has been a multi-use crop valued for its fiber, food, and medicinal uses. Various oriental and Asian cultures kept records of its production and numerous uses. Due to the similarities between industrial hemp (fiber and grain) and the narcotic/medical type of Cannabis, the production of industrial hemp was prohibited in most countries, wiping out centuries of learning and genetic resources. In the past two decades, most countries have legalized industrial hemp production, prompting a significant amount of research on the health benefits of hemp and hemp products. Current research is yet to verify the various health claims of the numerous commercially available hemp products. -

Technical Information
Real Food Blends™ Technical Information: Item 49748: Quinoa, Kale & Hemp Enteral Feeding Liquid Meal Real Food Blends™ Quinoa Kale & Hemp is a pre-packaged pureed food meal that is fed via feeding tube. Each 9.4-ounce pouch yields an 8-fluid ounce serving Description (237ml) delivering 11 grams protein, 19 grams fat and 32 grams of carbohydrate in 330 calories Water, kale, grape juice concentrate, hemp powder, extra virgin olive oil, quinoa, Ingredients cinnamon 13% calories from protein Caloric Distribution 38.5% calories from carbohydrate 48.5% calories from fat Protein Source Hemp powder, Quinoa, Kale Carbohydrate Source Grape juice concentrate, Quinoa, Hemp powder, Kale Fat Source Olive oil Water Content 84% Fiber / Source 4g per serving / hemp, cinnamon, kale, quinoa No Allergens • Manufactured in a facility that processes: egg, fish, wheat, milk, shellfish, soy, and tree nuts (almonds) Gluten Free Not tested. Does not contain gluten ingredients. Lactose Content 0 Phenylalanine 146 mg/ serving • Product is made from usual and customary foods. An individual would not be expected to have a reaction unless they were sensitive, or allergic, to an Contraindications ingredient. • Drug/nutrient interactions should be evaluated by the physician and/or pharmacist. Kosher No Halal Certified No Use as directed By your Health Care Professional • Bolus - Packaged ready to use. May consume orally. • Gravity or pump administration - Add 2-4 ounces of liquid as needed to User Instruction reduce viscosity. • Refrigerate any unused portion and use within 24 hours. • Flush tube with water after each feeding. May help address feeding intolerance for those exhiBiting the following symptoms: Nutritional Applications • Gagging/Retching • Constipation • Diarrhea • Vomiting • Failure to thrive • Bloating/Distension Free from Soy, corn, dairy, peanuts, and artificial color/flavor Shelf Life 3 years from date of manufacture, unopened, at room temperature As with any real food product, variations in caloric and nutrient levels should be expected due to growing conditions. -

Preventing the Use of Marijuana: Focus on Women and Pregnancy
EVIDENCE-BASED RESOURCE GUIDE SERIES Preventing the Use of Marijuana: Focus on Women and Pregnancy Acknowledgments This report was prepared for the Substance Abuse and Mental Health Services Administration (SAMHSA) under contract number HHSS2832017000651I/HHSS28342001T with SAMHSA. Thomas Clarke served as contracting officer representative. Disclaimer The views, opinions, and content of this publication are those of the authors and do not necessarily reflect the views, opinions, or policies of SAMHSA. Nothing in this document constitutes a direct or indirect endorsement by SAMHSA of any non-federal entity’s products, services, or policies, and any reference to non-federal entity’s products, services, or policies should not be construed as such. Public Domain Notice All material appearing in this publication is in the public domain and may be reproduced or copied without permission from SAMHSA. Citation of the source is appreciated. However, this publication may not be reproduced or distributed for a fee without the specific, written authorization of the Office of Communications, SAMHSA. Electronic Access This publication may be downloaded from http://store.samhsa.gov Recommended Citation Substance Abuse and Mental Health Services Administration (SAMHSA). Preventing the Use of Marijuana: Focus on Women and Pregnancy. SAMHSA Publication No. PEP19-PL-Guide-2 Rockville, MD: National Mental Health and Substance Use Policy Laboratory. Substance Abuse and Mental Health Services Administration, 2019. Originating Office National Mental Health and Substance Use Policy Laboratory, Substance Abuse and Mental Health Services Administration, 5600 Fishers Lane, Rockville, MD 20857, SAMHSA Publication No. PEP19-PL-Guide-2. Nondiscrimination Notice SAMHSA complies with applicable federal civil rights laws and does not discriminate on the basis of race, color, national origin, age, disability, or sex. -

2019 Update on Cannabidiol and Hemp Products
2019 Update on Cannabidiol and Hemp Products Presented by: Charles LoDico, M.S., F-ABFT Senior Chemist/Toxicologist Division of Workplace Programs Drug Testing Advisory Board June 11 , 2019 1 Presentation Objectives • Overview Marijuana • Potency • Scheduling and Research, • Policy • Commercialization of Marijuana 2 Marijuana Contains Over 400 Chemical Compounds: • 110 + Cannabinoids • Δ 9-THC (psychoactive) • CBD (non-psychoactive) • 200 + Terpenes (odor) • Flavonoids (color) 3 Trichome of Marijuana 4 Cultivated Throughout Recorded History Different Uses: • Industrial Fiber (rope, clothes) • Seed Oil (hemp oil) • Food (ground hemp seed for flour) • Recreation ( “high”) • Religious Customs (native cultures in rituals) • Medicine (Marinol®,and Epidiolex® (CBD)) 5 Cannabis Sativa L Cannabis – Hemp - Marijuana Commercial Hemp “Medical”/ Recreational Hemp CBD CBD CBD CBD CBD CBD CBD CBD CBD CBD CBD 6 Structural Similarity of CBD and Δ9-THC Chemistry Δ 9-THC CBD Molar Mass 314.469 Molar Mass 314.464 C-21 H-30 O-2 C-21 H-30 O-2 7 Potency: The New Normal • 1980s Marijuana: 4% THC • Mexican Marijuana: 6 - 11% THC • Current 2019 THC: 13 - 20% THC • Hashish/Hashish Oil: 20 - 40% THC • New Concentrates: 40 - 80% THC 8 Average THC and CBD % Marijuana Seizures 1995 – 2014 9 University of Mississippi, Potency MonitoringProgram Marijuana’s Acute Effects Cognition – Impaired short-term memory – Difficulty with complex tasks – Difficulty Learning Executive Function – Impaired decision-making – Increased risky behavior Mood (especially after high doses or edibles) – Anxiety – panic attacks – Psychosis - paranoia 10 List of Known Marijuana Risk’s • Psychosis • Agitated Delirum • Suicides • Seizures • Drugged Driving • Cannabinoid Hyperemesis Syndrome • Cardiovascular Effects • Pulmonary Affects • Contaminant Infections • Allergic Reactions • Anti-Coagulant effects due to contaminants 11 Consuming Marijuana • Smoking – Marijuana is absorbed through the capillaries in the lungs.