Cervical Sympathetic Trunk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variations of Thoracic Splanchnic Nerves and Its Clinical Implications
Int. J. Morphol., 23(3):247-251, 2005. Variations of Thoracic Splanchnic Nerves and its Clinical Implications Variaciones de los Nervios Esplácnicos Torácicos y sus Implicancias Clínicas *Tony George Jacob; ** Surbhi Wadhwa; ***Shipra Paul & ****Srijit Das JACOB, G. T.; WADHWA, S.; PAUL, S. & DAS, S. Variations of thoracic splanchnic nerves and its clinical implications. Int. J. Morphol., 23(3):247-251, 2005. SUMMARY:The present study reports an anomalous branching pattern of the thoracic sympathetic chain. At the level of T3 ganglion, an anomalous branch i.e accessory sympathetic chain (ASC) descended anteromedial to the main sympathetic chain (MSC). The MSC and the ASC communicated with each other at the level of T9, T10 and T11 ganglion, indicating the absence of classical pattern of greater, lesser and least splanchnic nerves on the right side. However, on the left side, the sympathetic chain displayed normal branching pattern. We opine that the ASC may be representing a higher origin of greater splanchnic nerve at the level of T3 ganglion and the branches from MSC at T9, T10 and T11 ganglion may be the lesser and least splanchnic nerves, which further joined the ASC (i.e presumably the greater splanchnic nerve) to form a common trunk. This common trunk pierced the right crus of diaphragm to reach the right suprarenal plexus after giving few branches to the celiac plexus. Awareness and knowledge of such anatomical variants of thoracic sympathetic chain may be helpful to surgeons in avoiding any incomplete denervation or preventing any inadvertent injury during thoracic sympathectomy. KEY WORDS: Splanchnic nerves; Sympathetic chain; Trunk thoracic; Ganglion. -

Of the Pediatric Mediastinum
MRI of the Pediatric Mediastinum Dianna M. E. Bardo, MD Director of Body MR & Co-Director of the 3D Innovation Lab Disclosures Consultant & Speakers Bureau – honoraria Koninklijke Philips Healthcare N V Author – royalties Thieme Publishing Springer Publishing Mediastinum - Anatomy Superior Mediastinum thoracic inlet to thoracic plane thoracic plane to diaphragm Inferior Mediastinum lateral – pleural surface anterior – sternum posterior – vertebral bodies Mediastinum - Anatomy Anterior T4 Mediastinum pericardium to sternum Middle Mediastinum pericardial sac Posterior Mediastinum vertebral bodies to pericardium lateral – pleural surface superior – thoracic inlet inferior - diaphragm Mediastinum – MR Challenges Motion Cardiac ECG – gating/triggering Breathing Respiratory navigation Artifacts Intubation – LMA Surgical / Interventional materials Mediastinum – MR Sequences ECG gated/triggered sequences SSFP – black blood SE – IR – GRE Non- ECG gated/triggered sequences mDIXON (W, F, IP, OP), eTHRIVE, turbo SE, STIR, DWI Respiratory – triggered, radially acquired T2W MultiVane, BLADE, PROPELLER Mediastinum – MR Sequences MRA / MRV REACT – non Gd enhanced Gd enhanced sequences THRIVE, mDIXON, mDIXON XD Mediastinum – Contents Superior Mediastinum PVT Left BATTLE: Phrenic nerve Vagus nerve Structures at the level of the sternal angle Thoracic duct Left recurrent laryngeal nerve (not the right) CLAPTRAP Brachiocephalic veins Cardiac plexus Aortic arch (and its 3 branches) Ligamentum arteriosum Thymus Aortic arch (inner concavity) Trachea Pulmonary -
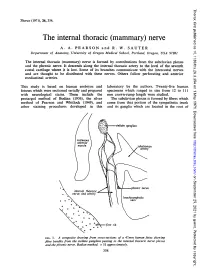
The Internal Thoracic (Mammary) Nerve A
Thorax: first published as 10.1136/thx.26.3.354 on 1 May 1971. Downloaded from Thorax (1971), 26, 354. The internal thoracic (mammary) nerve A. A. PEARSON and R. W. SAUTER Department of Anatomy, University of Oregon Medical School, Portland, Oregon, USA 97201 The internal thoracic (mammary) nerve is formed by contributions from the subclavian plexus and the phrenic nerve. It descends along the internal thoracic artery to the level of the seventh costal cartilage where it is lost. Some of its branches communicate with the intercostal nerves -and are thought to be distributed with these nerves. Others follow perforating and anterior mediastinal arteries. This study is based on human embryos and laboratory by the authors. Twenty-five human fetuses which were sectioned serially and prepared specimens which ranged in size from 12 to 111 with neurological stains. These include the mm crown-rump length were studied. protargol method of Bodian (1936), the silver The subclavian plexus is formed by fibres which method of Pearson and Whitlock (1949), and come from that portion of the sympathetic trunk other staining procedures developed in this and its ganglia which are located in the root of http://thorax.bmj.com/ on September 25, 2021 by guest. Protected copyright. FIG. 1. A composite drawing from cross-sections of a 47mm human fetus showing fibre bundles from the stellate ganglion passing to the internal thoracic nerve plexus and the phrenic nerve. Bodian method. x 51 approximately. 354 Thorax: first published as 10.1136/thx.26.3.354 on 1 May 1971. Downloaded from FIG. -

Sympathetic Control of Lower Esophageal Sphincter Function in the Cat: ACTION of DIRECT CERVICAL and SPLANCHNIC NERVE STIMULATION
Sympathetic Control of Lower Esophageal Sphincter Function in the Cat: ACTION OF DIRECT CERVICAL AND SPLANCHNIC NERVE STIMULATION Jacques Fournet, … , William J. Snape Jr., Sidney Cohen J Clin Invest. 1979;63(4):562-570. https://doi.org/10.1172/JCI109337. The purpose of this study was to determine the effect of direct stimulation of the sympathetic nerves on the lower esophageal sphincter (LES) in the anesthetized cat. Neither unilateral nor bilateral cervical sympathectomy, or splanchnicectomy significantly modified basal LES pressure in animals with intact vagi, or animals having undergone bilateral cervical vagotomy. Electrical stimulation of the cut, peripheral, cervical sympathetic trunk increased mean arterial blood pressure, but had no effect on LES pressure or LES relaxation as induced by vagal stimulation. Stimulation of the central end of the cervical sympathetic trunk had no effect on LES pressure. Stimulation of the central end of the cut splanchnic nerve produced a decrease in LES pressure with a maximal response of 69.1±16.0% (mean±SEM). This inhibitory response was not modified by either propranolol or bilateral cervical vagotomy. Stimulation of the peripheral end of the cut, greater splanchnic nerve gave an increase in LES pressure with a maximal response of 38.2±7.19 mm Hg. Guanethidine, in the presence or absence of the adrenal glands, significantly augmented this excitatory response. This response was also slightly increased by phentolamine alone at 10 V, 1 Hz, but was not altered by propranolol. The excitatory response was completely antagonized by atropine or by trimethaphan camsylate. Stimulation of the peripheral end of the splanchnic nerve inhibited LES relaxation as induced by […] Find the latest version: https://jci.me/109337/pdf Sympathetic Control of Lower Esophageal Sphincter Function in the Cat ACTION OF DIRECT CERVICAL AND SPLANCHNIC NERVE STIMULATION JACQUES FOURNET, WILLIAM J. -

Morphology of Sympathetic Chain in Saguinus Niger
Anais da Academia Brasileira de Ciências (2013) 85(1): 365-370 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 www.scielo.br/aabc Morphology of sympathetic chain in Saguinus niger MARINA P.E. PINTO1, ÉRIKA BRANCO1, EMERSON T. FIORETTO2, LUIZA C. PEREIRA3 and ANA R. LIMA1 1Universidade Federal Rural da Amazônia (UFRA), Instituto de Saúde e Produção Animal – ISPA, Faculdade de Medicina Veterinária, Avenida Perimetral, 2501, Belém, PA, Brasil 2 Universidade Federal de Sergipe (UFS), Cidade Universitária Professor José Aloísio de Campos, Avenida Marechal Rondon, s/n, Jardim Rosa Elze, São Cristovão, Aracajú, SE, Brasil 3 Empresa Hydro LTDA, Mina de Bauxita – Paragominas, PA, Brasil Manuscript received on March 20, 2012; accepted for publication on October 2, 2012 ABSTRACT Saguinus niger popularly known as Sauim, is a Brazilian North primate. Sympathetic chain investigation would support traumatic and/or cancer diagnosis which are little described in wild animals. The aim of this study was to describe the morphology and distribution of sympathetic chain in order to supply knowledge for neurocomparative research. Three female young animals that came death by natural causes were investigated. Animals were fixed in formaldehyde 10% and dissected along the sympathetic chain in neck, thorax and abdomen. Cranial cervical ganglion was located at the level of carotid bifurcation, related to carotid internal artery. In neck basis the vagosympathetic trunk divides into the sympathetic trunk and the parasympathetic vagal nerve. Sympathetic trunk ran in dorsal position and originated the stellate ganglia, formed by the fusion of caudal cervical and first thoracic ganglia. -

Mice Lack Carotid Body and Exhibit Abnormalities of the Superior
Available online at www.sciencedirect.com Developmental Biology 314 (2008) 236–247 www.elsevier.com/developmentalbiology FRS2α2F/2F mice lack carotid body and exhibit abnormalities of the superior cervical sympathetic ganglion and carotid sinus nerve ⁎ Yoko Kameda a, , Masataka Ito b, Toshiyuki Nishimaki a, Noriko Gotoh c a Department of Anatomy, Kitasato University School of Medicine, Sagamihara, Kanagawa 228-8555, Japan b Department of Anatomy, National Defense Medical College, Tokorozawa 359-8513, Japan c Division of Genetics, Institute of Medical Science, University of Tokyo, Minato-ku 108-8639, Japan Received for publication 25 May 2007; revised 26 November 2007; accepted 4 December 2007 Available online 8 December 2007 Abstract The docking protein FRS2α is an important mediator of fibroblast growth factor (FGF)-induced signal transduction, and functions by linking FGF receptors (FGFRs) to a variety of intracellular signaling pathways. We show that the carotid body is absent in FRS2α2F/2F mice, in which the Shp2-binding sites of FRS2α are disrupted. We also show that the carotid body rudiment is not formed in the wall of the third arch artery in mutant embryos. In wild-type mice, the superior cervical ganglion of the sympathetic trunk connects to the carotid body in the carotid bifurcation region, and extends thick nerve bundles into the carotid body. In FRS2α2F/2F mice, the superior cervical ganglion was present in the lower cervical region as an elongated feature, but failed to undergo cranio-ventral migration. In addition, few neuronal processes extended from the ganglion into the carotid bifurcation region. The number of carotid sinus nerve fibers that reached the carotid bifurcation region was markedly decreased, and baroreceptor fibers belonging to the glossopharyngeal nerve were absent from the basal part of the internal carotid artery in FRS2α2F/2F mutant mice. -

THE ANATOMY of the SYMPATHETHIC TRUNKS in MAN by MARTIN WRETE Histological Department, the University of Uppsala, Sweden
[ 448 ] THE ANATOMY OF THE SYMPATHETHIC TRUNKS IN MAN BY MARTIN WRETE Histological Department, The University of Uppsala, Sweden INTRODUCTION Even a cursory study of the anatomical descriptions of the cervical parts of the sympathetic trunks given in modern text-books or articles discloses that, now as earlier, great confusion exists with respect to terminology. This applies even to monographs and more specialized presentations. The primary cause of this confusion is the very marked variability of the trunks in the neck region, which gives wide scope for arbitrary interpretations of the arrangement; some uncertainty about the terminology and notation of other parts of the trunks also persists. It is true that the terms to be used for the sympathetic nervous system were fixed by the International Anatomical Nomenclature Committee (Nomina Anatomica, Paris, 1955). This does not, however, prevent some of the individual terms being used to denote different anatomical units, and for practical reasons (such as limiting printing costs) comprehensive explanations could not always be given in the annota- tions to the Parisian Nomina Anatomica. As one of the three members of the Sub- Committee responsible for the nomenclature of the peripheral nervous system, I wish to define more exactly my views on the terminology adopted for the sympathetic trunks. I also take this opportunity of revising a few terms I used in certain papers published some twenty years ago. In Nomina Anatomica the term truncus sympathicus is followed by the names of its ganglia, ganglia trunci sympathici, as well as of its connecting rami interganglio- nares. But, also under the heading ganglia trunci sympathici, the term ganglia intermedia is used to denote ganglia on the rami communicantes and certain ganglia on the trunks in the rami interganglionares between the other ganglia-namely the ganglion cervicale superius, ganglion cervicale medium, ganglion cervicothoracicum (s. -

Superior and Posterior Mediastina Reading: 1. Gray's Anatomy For
Dr. Weyrich G07: Superior and Posterior Mediastina Reading: 1. Gray’s Anatomy for Students, chapter 3 Objectives: 1. Subdivisions of mediastinum 2. Structures in Superior mediastinum 3. Structures in Posterior mediastinum Clinical Correlate: 1. Aortic aneurysms Superior Mediastinum (pp.181-199) 27 Review of the Subdivisions of the Mediastinum Superior mediastinum Comprises area within superior thoracic aperture and transverse thoracic plane -Transverse thoracic plane – arbitrary line from the sternal angle anteriorly to the IV disk or T4 and T5 posteriorly Inferior mediastinum Extends from transverse thoracic plane to diaphragm; 3 subdivisions Anterior mediastinum – smallest subdivision of mediastinum -Lies between the body of sternum and transversus thoracis muscles anteriorly and the pericardium posteriorly -Continuous with superior mediastinum at the sternal angle and limited inferiorly by the diaphragm -Consists of sternopericardial ligaments, fat, lymphatic vessels, and branches of internal thoracic vessels. Contains inferior part of thymus in children Middle mediastinum – contains heart Posterior mediastinum Superior Mediastinum Thymus – lies posterior to manubrium and extends into the anterior mediastinum -Important in development of immune system through puberty -Replaced by adipose tissue in adult Arterial blood supply -Anterior intercostals and mediastinal branches of internal thoracic artery Venous blood supply -Veins drain into left brachiocephalic, internal thoracic, and thymic veins 28 Brachiocephalic Veins - Formed by the -
Autonomic Nervous System
NERVOUS SYSTEM OUTLINE 18.1 Comparison of the Somatic and Autonomic Nervous Systems 540 18.2 Overview of the Autonomic Nervous System 542 18 18.3 Parasympathetic Division 545 18.3a Cranial Nerves 545 18.3b Sacral Spinal Nerves 545 18.3c Effects and General Functions of the Parasympathetic Division 545 Autonomic 18.4 Sympathetic Division 547 18.4a Organization and Anatomy of the Sympathetic Division 547 18.4b Sympathetic Pathways 550 Nervous 18.4c Effects and General Functions of the Sympathetic Division 550 18.5 Other Features of the Autonomic Nervous System 552 System 18.5a Autonomic Plexuses 552 18.5b Neurotransmitters and Receptors 553 18.5c Dual Innervation 554 18.5d Autonomic Reflexes 555 18.6 CNS Control of Autonomic Function 556 18.7 Development of the Autonomic Nervous System 557 MODULE 7: NERVOUS SYSTEM mck78097_ch18_539-560.indd 539 2/14/11 3:46 PM 540 Chapter Eighteen Autonomic Nervous System n a twisting downhill slope, an Olympic skier is concentrat- Recall from figure 14.2 (page 417) that the somatic nervous O ing on controlling his body to negotiate the course faster than system and the autonomic nervous system are part of both the anyone else in the world. Compared to the spectators in the viewing central nervous system and the peripheral nervous system. The areas, his pupils are more dilated, and his heart is beating faster SNS operates under our conscious control, as exemplified by vol- and pumping more blood to his skeletal muscles. At the same time, untary activities such as getting out of a chair, picking up a ball, organ system functions not needed in the race are practically shut walking outside, and throwing the ball for the dog to chase. -

Synchronized Electrical Stimulation of the Sympathetic and Parasympathetic Innervation of Bladder: Facili Tation of Initiation of Micturition in Dog
The Journal of UROLOGY Editor John T. Grayhack 1120 North Charles Street Baltimore, Maryland 21201 Associate Editor Associate Editor Terry D. Allen Jay Y. Gillenwater Dallas, Texas Charlottesville, Virginia Section Editor Section Editor Stuart S. Howards Patrick C. Walsh Charlottesville, Virginia Baltimore, Maryland EDITORIAL BOARD Mid-Atlantic New England New York North Central Patrick C. Walsh Bernard Lytton Michael J. Droller Joseph W. Segura Baltimore, Maryland New Haven, Connecticut New York, New York Rochester, Minnesota Northeastern South Central Southeastern Western Abraham Τ. K. Cockett Robert E. Donohue Floyd A. Fried Duncan E. Govan Rochester, New York Denver, Colorado Chapel Hill, North Carolina Stanford, California BOARD OF CONSULTANTS Marc Garnick Ryoichi Oyasu Boston, Massachusetts Chicago, Illinois Allyn W. Kimball Howard Pollack Baltimore, Maryland Cheltenham, Pennsylvania Bruce McClennan William U. Shipley St. Louis, Missouri Boston, Massachusetts William Murphy Lyn wood H. Smith, Jr. Memphis, Tennessee Rochester, Minnesota FORMER EDITORS Hugh H. Young J. A. Campbell Colston Hugh J. Jewett William W. Scott Herbert Brendler 1917-1945 1945-1966 1966-1977 1977-1983 1983-1985 The Journal of Urology (ISSN 0022-5347) is the Official Journal of the American Urological Association, Inc., and is published monthly by Williams & Wilkins, 428 East Preston Street, Baltimore, MD 21202. Second class postage paid at Baltimore, MD, and at additional mailing offices. Subscription rates $150.00 ($215.00 foreign); institutions $170.00 ($235.00 foreign); in-training $75.00 ($140.00 foreign); single copy $22.00 ($27.00 foreign). Subscription prices subject to change. To order call 1-800-638-6423 from anywhere in the U.S.; in Maryland call 1-800-638-4007. -

The Anatomy of Spinal Sympathetic Structures in the Cat
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 1978 The Anatomy of Spinal Sympathetic Structures in the Cat Kyungsoon Chung Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Anatomy Commons Recommended Citation Chung, Kyungsoon, "The Anatomy of Spinal Sympathetic Structures in the Cat" (1978). Dissertations. 1725. https://ecommons.luc.edu/luc_diss/1725 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1978 Kyungsoon Chung ; THE ANATOMY OF SPINAL SYMPATHETIC STRUCTURES IN THE CAT by Kyungsoon Chung A Dissertation Submitted to the Faculty of the Graduate School of Loyola University of Chicago in Partial Fulfillemnt of the Requirements for the Degree of Doctor of Philosophy April 1978 Dedicated to my parents, Mom and Dad ii ACKNOWLEDGEMENTS I would like to express my sincere appreciation to my adviser, Dr. Faith LaVelle, who gave unsparingly of her time and energy to assist in the fruition of this study. The guidance and comments of Dr. Robert Wurster throughout this study were specially appreciated. I would like to thank the faculty of the Depart- ment of Anatomy for giving me a chance for graduate study and for molding me as a scientist. This dissertation would not have been possible without the help and understanding of my husband and colleague, Jin Mo. -
Peripheral Nervous System 2: the Autonomic System 16 August 2010
Peripheral Nervous System 2: The Autonomic System 16 August 2010 Handout download: Blackboard or http://www.oucom.ohiou.edu/ dbms-witmer/anatomy_immersion.htm Reading: Moore’s COA6 57–65 Lawrence M. Witmer, PhD Professor of Anatomy Dept of Biomedical Sciences College of Osteopathic Medicine Ohio University Athens, Ohio 45701 [email protected] Somatic vs. Visceral attribute Somatic System Visceral System embryological “body wall:” somatic (parietal) “organs:” splanchnic origin of tissue mesoderm (dermatome, (visceral) mesoderm, myotome) endoderm examples of dermis of skin, skeletal muscles, glands, cardiac muscle, adult tissues connective tissues smooth muscle perception conscious, voluntary unconscious, involuntary Langman’s Embryo 9 2004 Sensory/Motor + Somatic/Visceral Somatic Visceral Sensory somatic sensory visceral sensory (Afferent) [General Somatic [General Visceral Afferent (GSA)] Afferent (GVA)] Motor somatic motor visceral motor (Efferent) [General Somatic [General Visceral Efferent (GSE)] Efferent (GVE)] Somatic Autonomic Nervous Nervous System System (Aug 2) (today) Overview of the Autonomic Nervous System Similarities between Sympathetic & Parasympathetic • Both are efferent (motor) systems: “visceromotor” • Both involve regulation of the “internal” environment generally outside of our conscious control: “autonomous” • Both involve 2 neurons that synapse in a peripheral ganglion • Innervate glands, smooth muscle, cardiac muscle glands CNS ganglion smooth muscle cardiac preganglionic postganglionic muscle neuron neuron Overview