Rho Gtpase Signalling Pathways in the Morphological Changes Associated with Apoptosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Maintenance of Epidermal Stem Cells in Skin Adnexa
International Journal of Molecular Sciences Review Development and Maintenance of Epidermal Stem Cells in Skin Adnexa Jaroslav Mokry * and Rishikaysh Pisal Medical Faculty, Charles University, 500 03 Hradec Kralove, Czech Republic; [email protected] * Correspondence: [email protected] Received: 30 October 2020; Accepted: 18 December 2020; Published: 20 December 2020 Abstract: The skin surface is modified by numerous appendages. These structures arise from epithelial stem cells (SCs) through the induction of epidermal placodes as a result of local signalling interplay with mesenchymal cells based on the Wnt–(Dkk4)–Eda–Shh cascade. Slight modifications of the cascade, with the participation of antagonistic signalling, decide whether multipotent epidermal SCs develop in interfollicular epidermis, scales, hair/feather follicles, nails or skin glands. This review describes the roles of epidermal SCs in the development of skin adnexa and interfollicular epidermis, as well as their maintenance. Each skin structure arises from distinct pools of epidermal SCs that are harboured in specific but different niches that control SC behaviour. Such relationships explain differences in marker and gene expression patterns between particular SC subsets. The activity of well-compartmentalized epidermal SCs is orchestrated with that of other skin cells not only along the hair cycle but also in the course of skin regeneration following injury. This review highlights several membrane markers, cytoplasmic proteins and transcription factors associated with epidermal SCs. Keywords: stem cell; epidermal placode; skin adnexa; signalling; hair pigmentation; markers; keratins 1. Epidermal Stem Cells as Units of Development 1.1. Development of the Epidermis and Placode Formation The embryonic skin at very early stages of development is covered by a surface ectoderm that is a precursor to the epidermis and its multiple derivatives. -

The Wiskott-Aldrich Syndrome: the Actin Cytoskeleton and Immune Cell Function
Disease Markers 29 (2010) 157–175 157 DOI 10.3233/DMA-2010-0735 IOS Press The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function Michael P. Blundella, Austen Wortha,b, Gerben Boumaa and Adrian J. Thrashera,b,∗ aMolecular Immunology Unit, UCL Institute of Child Health, London, UK bDepartment of Immunology, Great Ormond Street Hospital NHS Trust, Great Ormond Street, London, UK Abstract. Wiskott-Aldrich syndrome (WAS) is a rare X-linked recessive primary immunodeficiency characterised by immune dysregulation, microthrombocytopaenia, eczema and lymphoid malignancies. Mutations in the WAS gene can lead to distinct syndrome variations which largely, although not exclusively, depend upon the mutation. Premature termination and deletions abrogate Wiskott-Aldrich syndrome protein (WASp) expression and lead to severe disease (WAS). Missense mutations usually result in reduced protein expression and the phenotypically milder X-linked thrombocytopenia (XLT) or attenuated WAS [1–3]. More recently however novel activating mutations have been described that give rise to X-linked neutropenia (XLN), a third syndrome defined by neutropenia with variable myelodysplasia [4–6]. WASP is key in transducing signals from the cell surface to the actin cytoskeleton, and a lack of WASp results in cytoskeletal defects that compromise multiple aspects of normal cellular activity including proliferation, phagocytosis, immune synapse formation, adhesion and directed migration. Keywords: Wiskott-Aldrich syndrome, actin polymerization, lymphocytes, -

Keratin 19 Regulates Cell Shape and Cell-Cell Adhesion of MCF7 Cells While Maintaining E-Cadherin Localization at the Cell Surface
Keratin 19 regulates cell shape and cell-cell adhesion of MCF7 cells while maintaining E-cadherin localization at the cell surface Welcome to my poster. This is Sarah Alsharif, a PhD student from the biology department. I am glad to present the work our lab has been doing in the breast cancer field. In fact, after lung cancer, breast cancer is the second cause of death in women worldwide (1). It is estimated that every 18 seconds, approximately one new case of breast cancer is documented (2). No one dies due to cancer itself. The death is because of metastasis which takes place when cancer cells leave a breast in which they are formed and reach other sites such as the brain or lung. Our lab is interested in investigating the mechanism behind metastasis of breast cancer. Metastasis is associated with what is called epithelial to mesenchymal transition (EMT), the process characterized by loss of cell to cell adhesion and expression of epithelial markers such as keratin intermediate filament proteins, as you can see in the first three images of cells. Those filaments are keratins and they are critical for the shape and for maintaining mechanical integrity of epithelial cells via cell to cell complexes called desmosomes. Among different keratins, keratin 19 (K19) is highly expressed in many types of cancer including breast cancer, and is correlated with a worse prognosis (3). Consistently, K19 expression has been reported to be significantly higher in metastatic breast cancer tumor cells compared to primary tumors (4). The role of K19 on mechanical properties of cancer cells for cell migration and possible impact on metastasis in breast cancer patients is still unknown. -
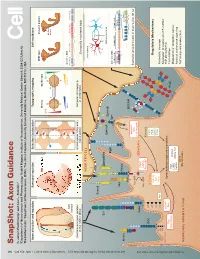
Snapshot: Axon Guidance Pasterkamp R
494 1 Cell Cell ??? SnapShot: Axon Guidance 153 SnapShot: XXXXXXXXXXXXXXXXXXXXXXXXXX 1 2 , ??MONTH?? ??DATE??, 200? ©200? Elsevier Inc. 200?©200? ElsevierInc. , ??MONTH?? ??DATE??, DOI R. Jeroen Pasterkamp and Alex L. Kolodkin , April11, 2013©2013Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.03.031 AUTHOR XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX 1 AFFILIATIONDepartment of XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Neuroscience and Pharmacology, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, 3584 CG Utrecht, The Netherlands; 2Department of Neuroscience, HHMI, The Johns Hopkins University School of Medicine, Baltimore, MD 21212, USA Axon attraction and repulsion Surround repulsion Selective fasciculation Topographic mapping Self-avoidance Wild-type Dscam1 mutant Sema3A A Retina SC/Tectum EphB EphrinB P D D Mutant T A neuron N P A V V Genomic DNA P EphA EphrinA Slit Exon 4 (12) Exon 6 (48) Exon 9 (33) Exon 17 (2) Netrin Commissural axon guidance Surround repulsion of peripheral Grasshopper CNS axon Retinotectal mapping at the CNS midline nerves in vertebrates fasciculation in vertebrates Drosophila mushroom body XXXXXXXXX NEURITE/CELL Isoneuronal Sema3 Slit Sema1/4-6 Heteroneuronal EphrinA EphrinB FasII Eph Genomic DNA Pcdh-α (14) Pcdh-β (22) Pcdh-γ (22) Variable Con Variable Con Nrp Plexin ** *** Netrin LAMELLIPODIA Con DSCAM ephexin Starburst amacrine cells in mammalian retina Ras-GTP Vav See online version for legend and references. α-chimaerin GEFs/GAPs Robo FARP Ras-GDP LARG RhoGEF Kinases DCC cc0 GTPases PKA cc1 FAK Regulatory Mechanisms See online versionfor??????. Cdc42 GSK3 cc2 Rac PI3K P1 Rho P2 cc3 Abl Proteolytic cleavage P3 Regulation of expression (TF, miRNA, FILOPODIA srGAP Cytoskeleton regulatory proteins multiple isoforms) Sos Trio Pcdh Cis inhibition DOCK180 PAK ROCK Modulation of receptors’ output LIMK Myosin-II Colin Forward and reverse signaling Actin Trafcking and endocytosis NEURONAL GROWTH CONE Microtubules SnapShot: Axon Guidance R. -

Microtubule and Cortical Forces Determine Platelet Size During Vascular Platelet Production
ARTICLE Received 5 Jan 2012 | Accepted 11 Apr 2012 | Published 22 May 2012 DOI: 10.1038/ncomms1838 Microtubule and cortical forces determine platelet size during vascular platelet production Jonathan N Thon1,2, Hannah Macleod1, Antonija Jurak Begonja2,3, Jie Zhu4, Kun-Chun Lee4, Alex Mogilner4, John H. Hartwig2,3 & Joseph E. Italiano Jr1,2,5 Megakaryocytes release large preplatelet intermediates into the sinusoidal blood vessels. Preplatelets convert into barbell-shaped proplatelets in vitro to undergo repeated abscissions that yield circulating platelets. These observations predict the presence of circular-preplatelets and barbell-proplatelets in blood, and two fundamental questions in platelet biology are what are the forces that determine barbell-proplatelet formation, and how is the final platelet size established. Here we provide insights into the terminal mechanisms of platelet production. We quantify circular-preplatelets and barbell-proplatelets in human blood in high-resolution fluorescence images, using a laser scanning cytometry assay. We demonstrate that force constraints resulting from cortical microtubule band diameter and thickness determine barbell- proplatelet formation. Finally, we provide a mathematical model for the preplatelet to barbell conversion. We conclude that platelet size is limited by microtubule bundling, elastic bending, and actin-myosin-spectrin cortex forces. 1 Hematology Division, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts 02115, USA. 2 Harvard Medical School, Boston, Massachusetts 02115, USA. 3 Translational Medicine Division, Brigham and Women’s Hospital, Boston, Massachusetts 02115, USA. 4 Department of Neurobiology, Physiology and Behavior and Department of Mathematics, University of California Davis, Davis, 95616, USA. 5 Vascular Biology Program, Department of Surgery, Children’s Hospital, Boston, Massachusetts 02115, USA. -

Table 2. Significant
Table 2. Significant (Q < 0.05 and |d | > 0.5) transcripts from the meta-analysis Gene Chr Mb Gene Name Affy ProbeSet cDNA_IDs d HAP/LAP d HAP/LAP d d IS Average d Ztest P values Q-value Symbol ID (study #5) 1 2 STS B2m 2 122 beta-2 microglobulin 1452428_a_at AI848245 1.75334941 4 3.2 4 3.2316485 1.07398E-09 5.69E-08 Man2b1 8 84.4 mannosidase 2, alpha B1 1416340_a_at H4049B01 3.75722111 3.87309653 2.1 1.6 2.84852656 5.32443E-07 1.58E-05 1110032A03Rik 9 50.9 RIKEN cDNA 1110032A03 gene 1417211_a_at H4035E05 4 1.66015788 4 1.7 2.82772795 2.94266E-05 0.000527 NA 9 48.5 --- 1456111_at 3.43701477 1.85785922 4 2 2.8237185 9.97969E-08 3.48E-06 Scn4b 9 45.3 Sodium channel, type IV, beta 1434008_at AI844796 3.79536664 1.63774235 3.3 2.3 2.75319499 1.48057E-08 6.21E-07 polypeptide Gadd45gip1 8 84.1 RIKEN cDNA 2310040G17 gene 1417619_at 4 3.38875643 1.4 2 2.69163229 8.84279E-06 0.0001904 BC056474 15 12.1 Mus musculus cDNA clone 1424117_at H3030A06 3.95752801 2.42838452 1.9 2.2 2.62132809 1.3344E-08 5.66E-07 MGC:67360 IMAGE:6823629, complete cds NA 4 153 guanine nucleotide binding protein, 1454696_at -3.46081884 -4 -1.3 -1.6 -2.6026947 8.58458E-05 0.0012617 beta 1 Gnb1 4 153 guanine nucleotide binding protein, 1417432_a_at H3094D02 -3.13334396 -4 -1.6 -1.7 -2.5946297 1.04542E-05 0.0002202 beta 1 Gadd45gip1 8 84.1 RAD23a homolog (S. -

Engineering of the Myosin-I Nucleotide-Binding
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 274, No. 44, Issue of October 29, pp. 31373–31381, 1999 © 1999 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Engineering of the Myosin-Ib Nucleotide-binding Pocket to Create Selective Sensitivity to N6-modified ADP Analogs* (Received for publication, April 30, 1999, and in revised form, July 21, 1999) Peter G. Gillespie‡§¶, Susan K. H. Gillespie‡i, John A. Mercer**, Kavita Shah‡‡, and Kevan M. Shokat‡‡ §§ From the Departments of ‡Physiology and §Neuroscience, The Johns Hopkins University, Baltimore, Maryland 21205, **McLaughlin Research Institute, Great Falls, Montana 59405, and the ‡‡Departments of Chemistry and Molecular Biology, Princeton University, Princeton, New Jersey 08544 Distinguishing the cellular functions carried out by and amplifies small movements within the head, and diverse enzymes of highly similar structure would be simplified tail domains that couple myosin molecules to other cellular by the availability of isozyme-selective inhibitors. To structures (2). determine roles played by individual members of the Although roles have been identified for conventional myosin large myosin superfamily, we designed a mutation in isozymes (the myosin-II class), elucidating cellular functions myosin’s nucleotide-binding pocket that permits bind- for unconventional myosin isozymes has been notably difficult. 6 ing of adenine nucleotides modified with bulky N sub- Cells may express a dozen or more myosin isozymes simulta- stituents. Introduction of this mutation, Y61G in rat my- neously (5, 6), and many of these isozymes have similar biochem- osin-Ib, did not alter the enzyme’s affinity for ATP or ical properties. -

Regulation of the Mammalian Target of Rapamycin Complex 2 (Mtorc2)
Regulation of the Mammalian Target Of Rapamycin Complex 2 (mTORC2) Inauguraldissertation Zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Klaus-Dieter Molle aus Heilbronn, Deutschland Basel, 2006 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät Auf Antrag von Prof. Michael N. Hall und Prof. Markus Affolter. Basel, den 21.11.2006 Prof. Hans-Peter Hauri Dekan Summary The growth controlling mammalian Target of Rapamycin (mTOR) is a conserved Ser/Thr kinase found in two structurally and functionally distinct complexes, mTORC1 and mTORC2. The tumor suppressor TSC1-TSC2 complex inhibits mTORC1 by acting on the small GTPase Rheb, but the role of TSC1-TSC2 and Rheb in the regulation of mTORC2 is unclear. Here we examined the role of TSC1-TSC2 in the regulation of mTORC2 in human embryonic kidney 293 cells. Induced knockdown of TSC1 and TSC2 (TSC1/2) stimulated mTORC2-dependent actin cytoskeleton organization and Paxillin phosphorylation. Furthermore, TSC1/2 siRNA increased mTORC2-dependent Ser473 phosphorylation of plasma membrane bound, myristoylated Akt/PKB. This suggests that loss of Akt/PKB Ser473 phosphorylation in TSC mutant cells, as reported previously, is due to inhibition of Akt/PKB localization rather than inhibition of mTORC2 activity. Amino acids and overexpression of Rheb failed to stimulate mTORC2 signaling. Thus, TSC1-TSC2 also inhibits mTORC2, but possibly independently of Rheb. Our results suggest that mTORC2 hyperactivation may contribute to the pathophysiology of diseases such as cancer and Tuberous Sclerosis Complex. i Acknowledgement During my PhD studies in the Biozentrum I received a lot of support from many people around me who I mention here to express my gratefulness. -

The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination
The Journal of Neuroscience, August 31, 2011 • 31(35):12579–12592 • 12579 Cellular/Molecular The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination Junji Yamauchi,1,3,5 Yuki Miyamoto,1 Hajime Hamasaki,1,3 Atsushi Sanbe,1 Shinji Kusakawa,1 Akane Nakamura,2 Hideki Tsumura,2 Masahiro Maeda,4 Noriko Nemoto,6 Katsumasa Kawahara,5 Tomohiro Torii,1 and Akito Tanoue1 1Department of Pharmacology and 2Laboratory Animal Resource Facility, National Research Institute for Child Health and Development, Setagaya, Tokyo 157-8535, Japan, 3Department of Biological Sciences, Tokyo Institute of Technology, Midori, Yokohama 226-8501, Japan, 4IBL, Ltd., Fujioka, Gumma 375-0005, Japan, and 5Department of Physiology and 6Bioimaging Research Center, Kitasato University School of Medicine, Sagamihara, Kanagawa 252-0374, Japan In development of the peripheral nervous system, Schwann cells proliferate, migrate, and ultimately differentiate to form myelin sheath. In all of the myelination stages, Schwann cells continuously undergo morphological changes; however, little is known about their underlying molecular mechanisms. We previously cloned the dock7 gene encoding the atypical Rho family guanine-nucleotide exchange factor (GEF) and reported the positive role of Dock7, the target Rho GTPases Rac/Cdc42, and the downstream c-Jun N-terminal kinase in Schwann cell migration (Yamauchi et al., 2008). We investigated the role of Dock7 in Schwann cell differentiation and myelination. Knockdown of Dock7 by the specific small interfering (si)RNA in primary Schwann cells promotes dibutyryl cAMP-induced morpholog- ical differentiation, indicating the negative role of Dock7 in Schwann cell differentiation. It also results in a shorter duration of activation of Rac/Cdc42 and JNK, which is the negative regulator of myelination, and the earlier activation of Rho and Rho-kinase, which is the positive regulator of myelination. -

The Role of Vimentin Intermediate Filaments in Cortical and Cytoplasmic Mechanics
1562 Biophysical Journal Volume 105 October 2013 1562–1568 The Role of Vimentin Intermediate Filaments in Cortical and Cytoplasmic Mechanics Ming Guo,† Allen J. Ehrlicher,†{ Saleemulla Mahammad,jj Hilary Fabich,† Mikkel H. Jensen,†** Jeffrey R. Moore,** Jeffrey J. Fredberg,‡ Robert D. Goldman,jj and David A. Weitz†§* † ‡ School of Engineering and Applied Sciences, Program in Molecular and Integrative Physiological Sciences, School of Public Health, and § { Department of Physics, Harvard University, Cambridge, Massachusetts; Beth Israel Deaconess Medical Center, Boston, Massachusetts; jj Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago, Illinois; and **Department of Physiology and Biophysics, Boston University, Boston, Massachusetts ABSTRACT The mechanical properties of a cell determine many aspects of its behavior, and these mechanics are largely determined by the cytoskeleton. Although the contribution of actin filaments and microtubules to the mechanics of cells has been investigated in great detail, relatively little is known about the contribution of the third major cytoskeletal component, intermediate filaments (IFs). To determine the role of vimentin IF (VIF) in modulating intracellular and cortical mechanics, we carried out studies using mouse embryonic fibroblasts (mEFs) derived from wild-type or vimentinÀ/À mice. The VIFs contribute little to cortical stiffness but are critical for regulating intracellular mechanics. Active microrheology measurements using optical tweezers in living cells reveal that the presence of VIFs doubles the value of the cytoplasmic shear modulus to ~10 Pa. The higher levels of cytoplasmic stiffness appear to stabilize organelles in the cell, as measured by tracking endogenous vesicle movement. These studies show that VIFs both increase the mechanical integrity of cells and localize intracellular components. -

Hypomesus Transpacificus
Aquatic Toxicology 105 (2011) 369–377 Contents lists available at ScienceDirect Aquatic Toxicology jou rnal homepage: www.elsevier.com/locate/aquatox Sublethal responses to ammonia exposure in the endangered delta smelt; Hypomesus transpacificus (Fam. Osmeridae) ∗ 1 2 Richard E. Connon , Linda A. Deanovic, Erika B. Fritsch, Leandro S. D’Abronzo , Inge Werner Aquatic Toxicology Laboratory, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, University of California, Davis, California 95616, United States a r t i c l e i n f o a b s t r a c t Article history: The delta smelt (Hypomesus transpacificus) is an endangered pelagic fish species endemic to the Received 9 May 2011 Sacramento-San Joaquin Estuary in Northern California, which acts as an indicator of ecosystem health Received in revised form 29 June 2011 in its habitat range. Interrogative tools are required to successfully monitor effects of contaminants upon Accepted 2 July 2011 the delta smelt, and to research potential causes of population decline in this species. We used microarray technology to investigate genome-wide effects in fish exposed to ammonia; one of multiple contami- Keywords: nants arising from wastewater treatment plants and agricultural runoff. A 4-day exposure of 57-day Hypomesus transpacificus + old juveniles resulted in a total ammonium (NH4 –N) median lethal concentration (LC50) of 13 mg/L, Delta smelt Microarray and a corresponding un-ionized ammonia (NH3) LC50 of 147 g/L. Using the previously designed delta + Biomarker smelt microarray we assessed altered gene transcription in juveniles exposed to 10 mg/L NH4 –N from Ammonia this 4-day exposure. -

Rho Kinase Proteins—Pleiotropic Modulators of Cell Survival and Apoptosis
ANTICANCER RESEARCH 31: 3645-3658 (2011) Review Rho Kinase Proteins—Pleiotropic Modulators of Cell Survival and Apoptosis CATHARINE A. STREET1 and BRAD A. BRYAN2 1Ghosh Science and Technology Center, Department of Biology, Worcester State University, Worcester, MA, U.S.A.; 2Center of Excellence in Cancer Research, Department of Biomedical Sciences, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, El Paso, TX, U.S.A. Abstract. Rho kinase (ROCK) proteins are Rho-GTPase regulators of actin cytoskeletal dynamics, and therefore activated serine/threonine kinases that function as modulators control cell migration and motility (1). Specifically, ROCK of actin-myosin cytoskeletal dynamics via regulation of Lin11, proteins promote the formation of stress fibers and focal Isl-1 & Mec-3 domain (LIM) kinase, myosin light chain (MLC), adhesions (Figure 1), but have also been implicated in and MLC phosphatase. A strong correlation between diverse processes such as cell junction integrity and cell cytoskeletal rearrangements and tumor cell invasion, metastasis, cycle control (2). ROCK activity is responsible for and deregulated microenvironment interaction has been stabilization of actin microfilaments as well as promoting reported in the literature, and the utilization of pharmacological cellular contraction and cell substratum contact. ROCK inhibitors of ROCK signaling for the treatment of cancer is stimulates actin polymerization via an inhibitory actively being pursued by a number of pharmaceutical phosphorylation of the actin severing LIM kinase (Figure 2). companies. Indeed, in many preclinical models ROCK inhibitors ROCK promotes cellular contraction and attachment via an have shown remarkable efficacy in reducing tumor growth and activating phosphorylation of myosin light chain (MLC) to metastasis.