Title Systematic Studies of Two Japanese Brown Frogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Local Population Differentiation and Phylogenetic
Japanese Journal of Herpetology 17(3): 91-97., June 1998 (C)1998 by The HerpetologicalSociety of Japan Local Population Differentiation and Phylogenetic Relationships of Russian Brown Frog, Rana amurensis Inferred by Mitochon- drial Cytochrome b Gene Sequences (Amphibia, Ranidae) TOMOKO TANAKA-UENO, MASAFUMI MATSUI, TAKANORI SATO, SEN TAKENAKA AND OSAMU TAKENAKA Abstract: In order to assess local population differentiation and phylogenetic relation- ships of a Russian brown frog, Rana amurensis, the sequences of 587 base pairs of the mitochondrial cytochrome b genes are compared with seven species of Japanese and one species complex of Taiwanese brown frogs (R. pirica, R. ornativentris, R. dybowskii, R. japonica, R. okinavana, R. tagoi, R. tsushimensis, and the R. sauteri complex). Genetic differentiation between populations of R. amurensis from Sakhalin and the Maritime Territory was found to be minimal. The resultant phylogenetic tree indicates monophyly of brown frogs and earliest divergence of R. amurensis among all the brown frogs studied. For this reason, separation of R. amurensis from the R. japonica group is suggested, but separation of the R. sauteri complex as a distinct ge- nus or subgenus Pseudorana is not supported. Key words: Brown frog; Cytochrome b; Phylogeny; Pseudorana; Rana amurensis; Russia Rana amurensis Boulenger, 1886 occupies a ships of Japanese brown frogs have been report- very wide range, including western Siberia east ed by Nishioka et al. (1992) and Tanaka et al. to Sakhalin, northern and eastern Mongolia, (1996). In the present study, we first investigat- northeastern China and Korea, and regions ed the degree of genetic differentiation between north to bevond the Arctic Circle to 71°N populations of R, amurensis in Sakhalin and (Frost, 1985). -

Phylogenetic Relationships of Brown Frogs from Taiwan and Japan Assessed by Mitochondrial Cytochrome B Gene Sequences (Rana: Ranidae)
ZOOLOGICAL SCIENCE 15: 283–288 (1998) © 1998 Zoological Society of Japan Phylogenetic Relationships of Brown Frogs from Taiwan and Japan Assessed by Mitochondrial Cytochrome b Gene Sequences (Rana: Ranidae) Tomoko Tanaka-Ueno1*, Masafumi Matsui1, Szu-Lung Chen2, Osamu Takenaka3 and Hidetoshi Ota4 1Graduate School of Human and Environmental Studies, Kyoto University, Sakyo-ku, Kyoto 606-01, Japan 2Department of Zoology, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-01, Japan 3Primate Research Institute, Kyoto University, Inuyama, Aichi 484, Japan 4Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-01, Japan ABSTRACT—In order to assess phylogenetic relationships of Taiwanese brown frogs (Rana longicrus and the R. sauteri complex), the partial sequences (587 base pairs) of the mitochondrial cytochrome b genes were compared with six brown frogs from Japan (R. pirica, R. ornativentris, R. japonica, R. tagoi tagoi, R. tsushimensis, and R. okinavana). Resultant phylogenetic trees indicated a considerable genetic differentia- tion between R. longicrus and R. japonica in spite of their close morphological and ecological similarities. The R. sauteri complex includes two genetically distinct groups that are not consistent with current classifica- tion. One group including populations of Alishan (central Taiwan) and Sanyi (western Taiwan) seemed to be closest to R. tagoi and the presumptive common ancestor of these frogs is thought to have diverged very early. Another group including a population from Wulai (northern Taiwan) showed a sister relationship with R. tsushimensis and R. okinavana, both isolated on small islands of Japan. These Taiwanese and Japanese brown frogs as a whole form a monophyletic group, and separation of the R. -

Zootaxa,Paraphyly of Chinese Amolops (Anura, Ranidae) and Phylogenetic Position of The
Zootaxa 1531: 49–55 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Paraphyly of Chinese Amolops (Anura, Ranidae) and phylogenetic position of the rare Chinese frog, Amolops tormotus HONG-XIA CAI1, 2, JING CHE2, JUN-FENG PANG2, ER-MI ZHAO1,4& YA-PING ZHANG2, 3,4 1Key Laboratory of Bio-resources and Eco-environment (Ministry of Education), College of Life Sciences, Sichuan University, Chengdu, China, 610064 2Laboratory of Cellular and Molecular Evolution, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming, China, 650223 3Laboratory for Conservation and Utilization of Bio-resources, Yunnan University, Kunming, China, 650091 4Corresponding authors. E-mail: [email protected]; [email protected] Abstract In order to evaluate the five species groups of Chinese Amolops based on morphological characteristics, and to clarify the phylogenetic position of the concave-eared torrent frog Amolops tormotus, we investigated the phylogeny of Amolops by maximum parsimony, Bayesian Inference, and maximum likelihood methods using two mitochondrial DNA fragments (12S rRNA, 16S rRNA). Our results supported a sister group relationship of Amolops ricketti and Amolops hainanensis. However, the grouping of Amolops mantzorum and Amolops monticola needs to be resolved with more data. Amolops tormotus was nested in genus Odorrana. Thus, recognition of the A. tormotus group is unwarranted and A. tormotus should be referred to genus Odorrana as O. tormota. This species is the sister group of O. nasica plus O. versabilis. The new classification implies that the genus Wurana is to be considered as junior subjective synonym of Odorrana. -

Title Phylogenetic Relationships of the Chinese Brown Frogs (Genus Rana)
Phylogenetic Relationships of the Chinese Brown Frogs Title (Genus Rana) Inferred from Partial Mitochondrial 12S and 16S rRNA Gene Sequences� Che, Jing; Pang, Junfeng; Zhao, Er-mi; Matsui, Masafumi; Author(s) Zhang, Ya-ping Citation Zoological Science (2007), 24(1): 71-80 Issue Date 2007-01 URL http://hdl.handle.net/2433/85329 Right (c) 日本動物学会 / Zoological Society of Japan Type Journal Article Textversion publisher Kyoto University ZOOLOGICAL SCIENCE 24:71–80 (2007) © 2007 Zoological Society of Japan Phylogenetic Relationships of the Chinese Brown Frogs (Genus Rana) Inferred from Partial Mitochondrial 12S and 16S rRNA Gene Sequences Jing Che1,2, Junfeng Pang2, Er-mi Zhao1, Masafumi Matsui3 and Ya-ping Zhang2* 1College of Life Sciences, Sichuan University, Chengdu 610064, China 2Laboratory of Cellular and Molecular Evolution, Kunming Institute of Zoology, The Chinese Academy of Sciences, Kunming 650223, China 3Graduate School of Human and Environmental Studies, Kyoto University, Sakyo, Kyoto 606-8501, Japan Based on partial sequences of the 12S and 16S ribosomal RNA genes, we estimated phylogenetic relationships among brown frogs of the Rana temporaria group from China. From the phylogenetic trees obtained, we propose to include Rana zhengi in the brown frogs. Monophyly of the brown frogs was not unambiguously supported, but four well-supported clades (A, B, C, and D) always emerged, although relationships among them remained unresolved. Clade A contained brown frogs with 24 chromosomes and was split into two distinct subclades (Subclade A-1: R. chensinensis and R. huanrenensis; Subclade A-2: R. dybowskii). Polytomous relationships among populations of R. chensinensis and R. huanrenensis suggested the necessity of further taxonomic assessment. -

Download Download
HAMADRYAD Vol. 27. No. 2. August, 2003 Date of issue: 31 August, 2003 ISSN 0972-205X CONTENTS T. -M. LEONG,L.L.GRISMER &MUMPUNI. Preliminary checklists of the herpetofauna of the Anambas and Natuna Islands (South China Sea) ..................................................165–174 T.-M. LEONG & C-F. LIM. The tadpole of Rana miopus Boulenger, 1918 from Peninsular Malaysia ...............175–178 N. D. RATHNAYAKE,N.D.HERATH,K.K.HEWAMATHES &S.JAYALATH. The thermal behaviour, diurnal activity pattern and body temperature of Varanus salvator in central Sri Lanka .........................179–184 B. TRIPATHY,B.PANDAV &R.C.PANIGRAHY. Hatching success and orientation in Lepidochelys olivacea (Eschscholtz, 1829) at Rushikulya Rookery, Orissa, India ......................................185–192 L. QUYET &T.ZIEGLER. First record of the Chinese crocodile lizard from outside of China: report on a population of Shinisaurus crocodilurus Ahl, 1930 from north-eastern Vietnam ..................193–199 O. S. G. PAUWELS,V.MAMONEKENE,P.DUMONT,W.R.BRANCH,M.BURGER &S.LAVOUÉ. Diet records for Crocodylus cataphractus (Reptilia: Crocodylidae) at Lake Divangui, Ogooué-Maritime Province, south-western Gabon......................................................200–204 A. M. BAUER. On the status of the name Oligodon taeniolatus (Jerdon, 1853) and its long-ignored senior synonym and secondary homonym, Oligodon taeniolatus (Daudin, 1803) ........................205–213 W. P. MCCORD,O.S.G.PAUWELS,R.BOUR,F.CHÉROT,J.IVERSON,P.C.H.PRITCHARD,K.THIRAKHUPT, W. KITIMASAK &T.BUNDHITWONGRUT. Chitra burmanica sensu Jaruthanin, 2002 (Testudines: Trionychidae): an unavailable name ............................................................214–216 V. GIRI,A.M.BAUER &N.CHATURVEDI. Notes on the distribution, natural history and variation of Hemidactylus giganteus Stoliczka, 1871 ................................................217–221 V. WALLACH. -

Elevational Variation in Reproductive and Life History Traits of Sauter's
Zoological Studies 42(1): 193-202 (2003) Elevational Variation in Reproductive and Life History Traits of Sauter’s Frog Rana sauteri Boulenger, 1909 in Taiwan Su-Ju Lai1,2, Yeong-Choy Kam3 and Yao-Sung Lin1,* 1Department of Zoology, National Taiwan University, Taipei, Taiwan 106, R.O.C. 2Zoology Division, Taiwan Endemic Species Research Institute, Chichi, Nantou, Taiwan 552, R.O.C. 3Department of Biology, National Changhua University of Education, Changhua, Taiwan 500, R.O.C. (Accepted October 23, 2002) Su-Ju Lai, Yeong-Choy Kam and Yao-Sung Lin (2003) Elevational variation in reproductive and life history traits of Sauter’s frog Rana sauteri Boulenger, 1909 in Taiwan. Zoological Studies 42(1): 193-202. Sauter’s frog, Rana sauteri Boulenger, 1909 in the west-central part of the Central Mountain Range of Taiwan at eleva- tions of 300 to 2360 m showed obvious elevational clines in reproductive and life cycle traits. With an increase in elevation, the breeding season and the periods of calling, aggregation, and egg deposition of mature frogs shifted from fall and winter (Oct. to Dec.) to spring (May), while the breeding period decreased but the larval period increased. Also, adult females at high elevations were larger and produced smaller clutch sizes but larg- er eggs and tadpoles. The temperature experiment showed that low temperatures in winter at high elevations and high temperatures in summer at low elevations are the primary environmental factors that define the breed- ing success of the species. Rana sauteri has experienced long-term selection by environmental factors (e.g., temperature), resulting in populations at high elevations breeding in spring and populations at low elevations breeding in fall and winter to ensure that their tadpoles can grow and complete metamorphosis. -

The Spemann Organizer Meets the Anterior‐
The Japanese Society of Developmental Biologists Develop. Growth Differ. (2015) 57, 218–231 doi: 10.1111/dgd.12200 Original Article The Spemann organizer meets the anterior-most neuroectoderm at the equator of early gastrulae in amphibian species Takanori Yanagi,1,2† Kenta Ito,1,2† Akiha Nishihara,1† Reika Minamino,1,2 Shoko Mori,1 Masayuki Sumida3 and Chikara Hashimoto1,2* 1JT Biohistory Research Hall, 1-1 Murasaki-cho, Takatsuki, Osaka 569-1125, 2Department of Biological Sciences, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, and 3Institute for Amphibian Biology, Hiroshima University, Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8526, Japan The dorsal blastopore lip (known as the Spemann organizer) is important for making the body plan in amphibian gastrulation. The organizer is believed to involute inward and migrate animally to make physical contact with the prospective head neuroectoderm at the blastocoel roof of mid- to late-gastrula. However, we found that this physical contact was already established at the equatorial region of very early gastrula in a wide variety of amphibian species. Here we propose a unified model of amphibian gastrulation movement. In the model, the organizer is present at the blastocoel roof of blastulae, moves vegetally to locate at the region that lies from the blastocoel floor to the dorsal lip at the onset of gastrulation. The organizer located at the blastocoel floor con- tributes to the anterior axial mesoderm including the prechordal plate, and the organizer at the dorsal lip ends up as the posterior axial mesoderm. During the early step of gastrulation, the anterior organizer moves to estab- lish the physical contact with the prospective neuroectoderm through the “subduction and zippering” move- ments. -

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -
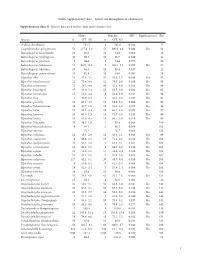
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

No Evidence for the 'Rate-Of-Living' Theory Across the Tetrapod Tree of Life
Received: 23 June 2019 | Revised: 30 December 2019 | Accepted: 7 January 2020 DOI: 10.1111/geb.13069 RESEARCH PAPER No evidence for the ‘rate-of-living’ theory across the tetrapod tree of life Gavin Stark1 | Daniel Pincheira-Donoso2 | Shai Meiri1,3 1School of Zoology, Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel Abstract 2School of Biological Sciences, Queen’s Aim: The ‘rate-of-living’ theory predicts that life expectancy is a negative function of University Belfast, Belfast, United Kingdom the rates at which organisms metabolize. According to this theory, factors that accel- 3The Steinhardt Museum of Natural History, Tel Aviv University, Tel Aviv, Israel erate metabolic rates, such as high body temperature and active foraging, lead to organismic ‘wear-out’. This process reduces life span through an accumulation of bio- Correspondence Gavin Stark, School of Zoology, Faculty of chemical errors and the build-up of toxic metabolic by-products. Although the rate- Life Sciences, Tel Aviv University, Tel Aviv, of-living theory is a keystone underlying our understanding of life-history trade-offs, 6997801, Israel. Email: [email protected] its validity has been recently questioned. The rate-of-living theory has never been tested on a global scale in a phylogenetic framework, or across both endotherms and Editor: Richard Field ectotherms. Here, we test several of its fundamental predictions across the tetrapod tree of life. Location: Global. Time period: Present. Major taxa studied: Land vertebrates. Methods: Using a dataset spanning the life span data of 4,100 land vertebrate spe- cies (2,214 endotherms, 1,886 ectotherms), we performed the most comprehensive test to date of the fundamental predictions underlying the rate-of-living theory. -

Salamander Species Listed As Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016
Salamander Species Listed as Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016 Effective January 28, 2016, both importation into the United States and interstate transportation between States, the District of Columbia, the Commonwealth of Puerto Rico, or any territory or possession of the United States of any live or dead specimen, including parts, of these 20 genera of salamanders are prohibited, except by permit for zoological, educational, medical, or scientific purposes (in accordance with permit conditions) or by Federal agencies without a permit solely for their own use. This action is necessary to protect the interests of wildlife and wildlife resources from the introduction, establishment, and spread of the chytrid fungus Batrachochytrium salamandrivorans into ecosystems of the United States. The listing includes all species in these 20 genera: Chioglossa, Cynops, Euproctus, Hydromantes, Hynobius, Ichthyosaura, Lissotriton, Neurergus, Notophthalmus, Onychodactylus, Paramesotriton, Plethodon, Pleurodeles, Salamandra, Salamandrella, Salamandrina, Siren, Taricha, Triturus, and Tylototriton The species are: (1) Chioglossa lusitanica (golden striped salamander). (2) Cynops chenggongensis (Chenggong fire-bellied newt). (3) Cynops cyanurus (blue-tailed fire-bellied newt). (4) Cynops ensicauda (sword-tailed newt). (5) Cynops fudingensis (Fuding fire-bellied newt). (6) Cynops glaucus (bluish grey newt, Huilan Rongyuan). (7) Cynops orientalis (Oriental fire belly newt, Oriental fire-bellied newt). (8) Cynops orphicus (no common name). (9) Cynops pyrrhogaster (Japanese newt, Japanese fire-bellied newt). (10) Cynops wolterstorffi (Kunming Lake newt). (11) Euproctus montanus (Corsican brook salamander). (12) Euproctus platycephalus (Sardinian brook salamander). (13) Hydromantes ambrosii (Ambrosi salamander). (14) Hydromantes brunus (limestone salamander). (15) Hydromantes flavus (Mount Albo cave salamander). -

(Amphibia: Ranidae) on Sumatra, Indonesia
Phylogenetic systematics, diversity, and biogeography of the frogs with gastromyzophorous tadpoles (Amphibia: Ranidae) on Sumatra, Indonesia Dissertation zur Erlangung des Doktorgrades Fachbereich Biologie An der Fakultät für Mathematik, Informatik und Naturwissenschaften der Universität Hamburg Vorgelegt von Umilaela Arifin Hamburg, 2018 Tag der Disputation: 25 January 2019 Folgende Gutachter empfehlen die Annahme der Dissertation: 1. Prof. Dr. Alexander Haas 2. Prof. Dr. Bernhard Hausdorf “To reach the same destination, some people might only need one step but some other people might need two, three, a hundred, or a thousand steps. Never give up! Some are successful because they work harder than other people, not because they are smart.” –dti- Preface Preface It is such a relief to have finally finished writing this dissertation entitled “Phylogenetic systematics, diversity, and biogeography of the frogs with gastromyzophorous tadpoles (Amphibia: Ranidae) on Sumatra, Indonesia”. Thank to Allah, who has always embraced me in any situation, especially during my doctoral studies. The work I have done over the past five years is dedicated not only to myself, but also to all the people, who came into my life for various reasons. Also, this thesis is my small contribution to Indonesia (the “Ibu Pertiwi”) and its fascinating biodiversity. I hope to continue actively contributing to the field of herpetology in the future, simply because it is my greatest passion! During my childhood, especially through my high school years, it never crossed my mind that I would end up becoming a scientist. Coming from an ordinary Indonesian family and living in a small town made my parents worry about the education their children would need, in order to have a better life in the future.