Role of the Fronto-Parietal Cortex in Prospective Action Judgments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Gliomas of the Cingulate Gyrus: Surgical Management and Functional Outcome
Neurosurg Focus 27 (2):E9, 2009 Gliomas of the cingulate gyrus: surgical management and functional outcome MAREC VON LEHE , M.D., AN D JOHANNES SCHRA mm , M.D. Neurochirurgische Klinik, Universitätsklinik Bonn, Germany Object. In this paper, the authors’ goal was to summarize their experience with the surgical treatment of gliomas arising from the cingulate gyrus. Methods. The authors analyzed preoperative data, surgical strategies, complications, and functional outcome in a series of 34 patients (mean age 42 years, range 12–69 years; 14 females) who underwent 38 operations between May 2001 and November 2008. Results. In 7 cases (18%) the tumor was located in the posterior (parietal) part of the cingulate gyrus, and in 31 (82%) the tumor was in the anterior (frontal) part. In 10 cases (26%) the glioma was solely located in the cingulate gyrus, and in 28 cases (74%) the tumor extended to the supracingular frontal/parietal cortex. Most cases (23 [61%]) had seizures as the presenting symptom, 8 patients (24%) suffered from a hemiparesis/hemihypesthesia, and 4 pa- tients (12%) had aphasic symptoms. The authors chose an interhemispheric approach for tumor resection in 11 (29%) and a transcortical approach in 27 (71%) cases; intraoperative electrophysiological monitoring was applied in 23 (61%) and neuronavigation in 15 (39%) cases. A > 90% resection was achieved in 32 (84%) and > 70% in another 5 (13%) cases. Tumors were classified as low-grade gliomas in 11 cases (29%). A glioblastoma multiforme (WHO Grade IV, 10 cases [26%]) and oligoastrocytoma (WHO Grade III, 9 cases [24%]) were the most frequent histopathological results. -
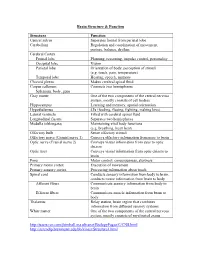
Brain Structure & Function Structure Function Central Sulcus Separates
Brain Structure & Function Structure Function Central sulcus Separates frontal from parietal lobe Cerebellum Regulation and coordination of movement, posture, balance, rhythm Cerebral Cortex Frontal lobe Planning, reasoning, impulse control, personality Occipital lobe Vision Parietal lobe Orientation of body, perception of stimuli (e.g. touch, pain, temperature) Temporal lobe Hearing, speech, memory Choroid plexus Makes cerebral spinal fluid Corpus callosum Connects two hemispheres Splenium, body, genu Gray matter One of the two components of the central nervous system, mostly consists of cell bodies Hippocampus Learning and memory, spatial orientation Hypothalamus 4Fs (feeding, fleeing, fighting, making love) Lateral ventricle Filled with cerebral spinal fluid Longitudinal fissure Separates two hemispheres Medulla (oblongata) Maintaining vital body functions (e.g. breathing, heart beat) Olfactory bulb Sense olfactory stimuli Olfactory nerve (Cranial nerve 1) Conveys olfactory information from nose to brain Optic nerve (Cranial nerve 2) Conveys visual information from eyes to optic chiasm Optic tract Conveys visual information from optic chiasm to brain Pons Motor control, consciousness, alertness Primary motor cortex Execution of movement Primary sensory cortex Processing information about touch Spinal cord Conducts sensory information from body to brain, conducts motor information from brain to body Afferent fibers Communicate sensory information from body to brain Efferent fibers Communicate muscle information from brain to body Thalamus Relay station, brain region that combines information from different sensory systems White matter One of the two components of the central nervous system, mostly consists of myelinated axons http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CNS.html http://serendip.brynmawr.edu/bb/kinser/Structure1.html . -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -

Alterations in Reward Network Functional Connectivity Are Associated with Increased Food Addiction in Obese Individuals Soumya Ravichandran1, Ravi R
www.nature.com/scientificreports OPEN Alterations in reward network functional connectivity are associated with increased food addiction in obese individuals Soumya Ravichandran1, Ravi R. Bhatt1,6, Bilal Pandit1, Vadim Osadchiy1,2, Anita Alaverdyan1, Priten Vora1, Jean Stains1,2,3, Bruce Nalibof1,2,3,4, Emeran A. Mayer1,2,3,4,5 & Arpana Gupta1,2,3,4* Functional neuroimaging studies in obesity have identifed alterations in the connectivity within the reward network leading to decreased homeostatic control of ingestive behavior. However, the neural mechanisms underlying sex diferences in the prevalence of food addiction in obesity is unknown. The aim of the study was to identify functional connectivity alterations associated with: (1) Food addiction, (2) Sex- diferences in food addiction, (3) Ingestive behaviors. 150 participants (females: N = 103, males: N = 47; food addiction: N = 40, no food addiction: N = 110) with high BMI ≥ 25 kg/m2 underwent functional resting state MRIs. Participants were administered the Yale Food Addiction Scale (YFAS), to determine diagnostic criteria for food addiction (YFAS Symptom Count ≥ 3 with clinically signifcant impairment or distress), and completed ingestive behavior questionnaires. Connectivity diferences were analyzed using a general linear model in the CONN Toolbox and images were segmented using the Schaefer 400, Harvard–Oxford Subcortical, and Ascending Arousal Network atlases. Signifcant connectivities and clinical variables were correlated. Statistical signifcance was corrected for multiple comparisons at q < .05. (1) Individuals with food addiction had greater connectivity between brainstem regions and the orbital frontal gyrus compared to individuals with no food addiction. (2) Females with food addiction had greater connectivity in the salience and emotional regulation networks and lowered connectivity between the default mode network and central executive network compared to males with food addiction. -

Mirror Neurons for Execution, Observation, and Imagery ⁎ Flavia Filimon,A, Jonathan D
www.elsevier.com/locate/ynimg NeuroImage 37 (2007) 1315–1328 Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery ⁎ Flavia Filimon,a, Jonathan D. Nelson,a,b Donald J. Hagler,a and Martin I. Serenoa aDepartment of Cognitive Science, University of California, San Diego, 9500 Gilman Dr. #0515, La Jolla, CA 92093-0515, USA bComputational Neurobiology Laboratory, Salk Institute, La Jolla, CA, USA Received 5 April 2007; revised 27 May 2007; accepted 8 June 2007 Available online 18 June 2007 We used functional magnetic resonance imaging (fMRI) to map the Electrophysiological studies in macaques have identified several cortical representations of executed reaching, observed reaching, and frontal areas involved in hand action representations (Preuss et al., imagined reaching in humans. Whereas previous studies have mostly 1996; Muakkassa and Strick, 1979; Matelli and Luppino, 2001; examined hand actions related to grasping, hand–object interactions, Rizzolatti et al., 1988). For instance, both dorsal premotor cortex or local finger movements, here we were interested in reaching only (i.e. (PMd, or F2 and F7) and ventral premotor cortex (PMv, or F4 and the transport phase of the hand to a particular location in space), F5) contain arm/hand representations that are specific to certain without grasping. We hypothesized that mirror neuron areas specific to reaching-related representations would be active in all three conditions. motor actions, such as grasping or reaching (Matelli and Luppino, An overlap between executed, observed, and imagined reaching 2001). More specifically, neurons that respond to both hand action activations was found in dorsal premotor cortex as well as in the execution (e.g. -

A Landmark for Identification of the Central Sulcus Using Functional Magnetic Resonance Imaging
The central sulcal vein: a landmark for identification of the central sulcus using functional magnetic resonance imaging Tarek Ahmed Yousry, M.D., Urs Dieter Schmid, M.D., Dorothea Schmidt, M.D., Thomas Hagen, M.D., Andre Jassoy, M.D., and Maximilian Friedrich Reiser, M.D. Department of Neuroradiology, and Departments of Diagnostic Radiology, Klinikum Grosshadern and Klinikum Innenstadt, Ludwig Maximilian University, Munich, Germany; Neurosurgical Unit, Klinik Im Park Zurich and Ludwig Maximilian University, Munich, Germany; and Department of Neuroradiology, Saarland University, Homburg-Saar, Germany The authors evaluated the anatomical location of the central sulcus (CS) in 24 cerebral hemispheres (eight in which tumors were located centrally, 16 in controls) using: 1) classic anatomical landmarks seen on magnetic resonance (MR) imaging (24 hemispheres); 2) functional MR imaging (24 hemispheres); and 3) intraoperative electrical stimulation mapping (eight hemispheres). On MR imaging the CS was identified with certainty in 79% of hemispheres (four of eight in patients, 15 of 16 in controls). Functional MR imaging identified a parenchymal "motor hand area" in only 83% (20 of 24 hemispheres; five of eight in patients, 15 of 16 in controls); this area was located in the precentral gyrus in 16 (80%) of 20, additionally in the postcentral gyrus in 10 (50%) of 20, and exclusively in the postcentral gyrus in four (20%) of 20. In contrast, functional MR imaging detected one to three sulcal veins presumably draining blood from the adjacent motor hand area in 100% (24 of 24) of the hemispheres studied, and anatomical MR imaging and intraoperative mapping localized these veins in the CS. -

Identifies the Central Sulcus on 3T High
Published December 8, 2016 as 10.3174/ajnr.A5014 ORIGINAL RESEARCH ADULT BRAIN The “White Gray Sign” Identifies the Central Sulcus on 3T High- Resolution T1-Weighted Images X O.F. Kaneko, X N.J. Fischbein, X J. Rosenberg, X M. Wintermark, and X M.M. Zeineh ABSTRACT BACKGROUND AND PURPOSE: The central sulcus is an important anatomic landmark, but most methods of identifying it rely on variable gyral and sulcal patterns. We describe and assess the accuracy of reduced gray-white contrast along the central sulcus, an observation we term the “white gray sign.” MATERIALS AND METHODS: We conducted a retrospective review of 51 fMRIs with a T1-weighted 3D inversion recovery fast-spoiled gradient-echo and concomitant hand-motor fMRI, which served as confirmation for the location of the central sulcus. To measure gray-white contrast across the central and adjacent sulci, we performed a quantitative analysis of 25 normal hemispheres along the anterior and posterior cortices and intervening white matter of the pre- and postcentral gyri. 3D inversion recovery fast-spoiled gradient-echo axial images from 51 fMRIs were then evaluated by 2 raters for the presence of the white gray sign as well as additional established signs of the central sulcus: the bracket, cortical thickness, omega, and T signs. RESULTS: The mean gray-white contrast along the central sulcus was 0.218 anteriorly and 0.237 posteriorly, compared with 0.320 and 0.295 along the posterior precentral and anterior postcentral sulci, respectively (PϽ .001). Both raters correctly identified the central sulcus in all 35 normal and 16 abnormal hemispheres.