Regional and Lobe Parcellation Rhesus Monkey Brain Atlas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Gliomas of the Cingulate Gyrus: Surgical Management and Functional Outcome
Neurosurg Focus 27 (2):E9, 2009 Gliomas of the cingulate gyrus: surgical management and functional outcome MAREC VON LEHE , M.D., AN D JOHANNES SCHRA mm , M.D. Neurochirurgische Klinik, Universitätsklinik Bonn, Germany Object. In this paper, the authors’ goal was to summarize their experience with the surgical treatment of gliomas arising from the cingulate gyrus. Methods. The authors analyzed preoperative data, surgical strategies, complications, and functional outcome in a series of 34 patients (mean age 42 years, range 12–69 years; 14 females) who underwent 38 operations between May 2001 and November 2008. Results. In 7 cases (18%) the tumor was located in the posterior (parietal) part of the cingulate gyrus, and in 31 (82%) the tumor was in the anterior (frontal) part. In 10 cases (26%) the glioma was solely located in the cingulate gyrus, and in 28 cases (74%) the tumor extended to the supracingular frontal/parietal cortex. Most cases (23 [61%]) had seizures as the presenting symptom, 8 patients (24%) suffered from a hemiparesis/hemihypesthesia, and 4 pa- tients (12%) had aphasic symptoms. The authors chose an interhemispheric approach for tumor resection in 11 (29%) and a transcortical approach in 27 (71%) cases; intraoperative electrophysiological monitoring was applied in 23 (61%) and neuronavigation in 15 (39%) cases. A > 90% resection was achieved in 32 (84%) and > 70% in another 5 (13%) cases. Tumors were classified as low-grade gliomas in 11 cases (29%). A glioblastoma multiforme (WHO Grade IV, 10 cases [26%]) and oligoastrocytoma (WHO Grade III, 9 cases [24%]) were the most frequent histopathological results. -

In the Motion Area of the Superior Temporal Sulcus Were Directionally Selective. 5
J. Phyeiol. (1978), 277, pp. 273-290 273 With 1 plate and 10 text-figurem Printed in Great Britain UNIFORMITY AND DIVERSITY OF STRUCTURE AND FUNCTION IN RHESUS MONKEY PRESTRIATE VISUAL CORTEX BY S. M. ZEKI* From the Department of Anatomy, University College London, London WC1E 6BT (Received 31 March 1977) SUMMARY 1. Recordings were made from single neurones, or small clusters of cells, in five prestriate visual areas of rhesus monkey cortex. The cells were studied for their binocularity, as well as for their orientational, motion and colour preferences. In all, 1500 cells were studied, 250 cells for each of the areas V2, V3, V3A and the motion area ofthe posterior bank ofthe superior temporal sulcus, and 500 cells for V4. All the cells referred to in this study can be placed in one prestriate area or another unambiguously. 2. The great majority of cells in all areas were binocularly driven, without mono- cular preferences. Within each area, there were cells that either preferred binocular stimulation markedly, or were responsive to binocular stimulation only. The ocular interaction histograms for all areas are remarkably similar when tested at a fixed disparity. 3. Over 70 % of the cells in areas V2, V3 and V3A were selective for orientation. The receptive fields of cells were larger in V3 and V3A than in V2. By contrast, less than 50 % of the cells in V4 and the motion area of the superior temporal sulcus were orientation selective. 4. Directionally selective cells were found in all areas. But they were present in small numbers (< 15 %) in areas V2, V3, V3A and V4. -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -
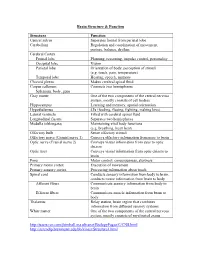
Brain Structure & Function Structure Function Central Sulcus Separates
Brain Structure & Function Structure Function Central sulcus Separates frontal from parietal lobe Cerebellum Regulation and coordination of movement, posture, balance, rhythm Cerebral Cortex Frontal lobe Planning, reasoning, impulse control, personality Occipital lobe Vision Parietal lobe Orientation of body, perception of stimuli (e.g. touch, pain, temperature) Temporal lobe Hearing, speech, memory Choroid plexus Makes cerebral spinal fluid Corpus callosum Connects two hemispheres Splenium, body, genu Gray matter One of the two components of the central nervous system, mostly consists of cell bodies Hippocampus Learning and memory, spatial orientation Hypothalamus 4Fs (feeding, fleeing, fighting, making love) Lateral ventricle Filled with cerebral spinal fluid Longitudinal fissure Separates two hemispheres Medulla (oblongata) Maintaining vital body functions (e.g. breathing, heart beat) Olfactory bulb Sense olfactory stimuli Olfactory nerve (Cranial nerve 1) Conveys olfactory information from nose to brain Optic nerve (Cranial nerve 2) Conveys visual information from eyes to optic chiasm Optic tract Conveys visual information from optic chiasm to brain Pons Motor control, consciousness, alertness Primary motor cortex Execution of movement Primary sensory cortex Processing information about touch Spinal cord Conducts sensory information from body to brain, conducts motor information from brain to body Afferent fibers Communicate sensory information from body to brain Efferent fibers Communicate muscle information from brain to body Thalamus Relay station, brain region that combines information from different sensory systems White matter One of the two components of the central nervous system, mostly consists of myelinated axons http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CNS.html http://serendip.brynmawr.edu/bb/kinser/Structure1.html . -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess. -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior