Alterations in Reward Network Functional Connectivity Are Associated with Increased Food Addiction in Obese Individuals Soumya Ravichandran1, Ravi R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Circular Representation of Human Cortical Networks for Subject and Population-Level Connectomic Visualization
NeuroImage 60 (2012) 1340–1351 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Full Length Articles Circular representation of human cortical networks for subject and population-level connectomic visualization Andrei Irimia ⁎, Micah C. Chambers, Carinna M. Torgerson, John D. Van Horn Laboratory of Neuro Imaging, Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles, 635 Charles E Young Drive South, Suite 225, Los Angeles, CA 90095, USA article info abstract Article history: Cortical network architecture has predominantly been investigated visually using graph theory representa- Received 13 September 2011 tions. In the context of human connectomics, such representations are not however always satisfactory Revised 19 January 2012 because canonical methods for vertex–edge relationship representation do not always offer optimal insight Accepted 20 January 2012 regarding functional and structural neural connectivity. This article introduces an innovative framework for Available online 28 January 2012 the depiction of human connectomics by employing a circular visualization method which is highly suitable to the exploration of central nervous system architecture. This type of representation, which we name a Keywords: ‘ ’ Connectomics connectogram , has the capability of classifying neuroconnectivity relationships intuitively and elegantly. A Cortical network multimodal protocol for MRI/DTI neuroimaging data acquisition is here combined with automatic image seg- DTI mentation to (1) extract cortical and non-cortical anatomical structures, (2) calculate associated volumetrics MRI and morphometrics, and (3) determine patient-specific connectivity profiles to generate subject-level and Neuroimaging population-level connectograms. The scalability of our approach is demonstrated for a population of 50 adults. -

Progress and Challenges in Probing the Human Brain
University of Pennsylvania ScholarlyCommons Neuroethics Publications Center for Neuroscience & Society 10-2015 Progress and Challenges in Probing the Human Brain Russell A. Poldrack Martha J. Farah University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/neuroethics_pubs Part of the Bioethics and Medical Ethics Commons, Neuroscience and Neurobiology Commons, and the Neurosciences Commons Recommended Citation Poldrack, R. A., & Farah, M. J. (2015). Progress and Challenges in Probing the Human Brain. Nature, 526 (7573), 371-379. http://dx.doi.org/10.1038/nature15692 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/neuroethics_pubs/136 For more information, please contact [email protected]. Progress and Challenges in Probing the Human Brain Abstract Perhaps one of the greatest scientific challenges is to understand the human brain. Here we review current methods in human neuroscience, highlighting the ways that they have been used to study the neural bases of the human mind. We begin with a consideration of different levels of description relevant to human neuroscience, from molecules to large-scale networks, and then review the methods that probe these levels and the ability of these methods to test hypotheses about causal mechanisms. Functional MRI is considered in particular detail, as it has been responsible for much of the recent growth of human neuroscience research. We briefly er view its inferential strengths and weaknesses and present examples of new analytic approaches that allow inferences beyond simple localization of psychological processes. Finally, we review the prospects for real-world applications and new scientific challenges for human neuroscience. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Evolutionary Expansion of Connectivity Between Multimodal Association Areas in the Human Brain Compared with Chimpanzees,” by Dirk Jan Ardesch, Lianne H
Correction NEUROSCIENCE Correction for “Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees,” by Dirk Jan Ardesch, Lianne H. Scholtens, Longchuan Li, Todd M. Preuss, James K. Rilling, and Martijn P. van den Heuvel, which was first published March 18, 2019; 10.1073/pnas.1818512116 (Proc Natl Acad Sci USA 116:7101–7106). The authors wish to note the following: “No new MRI data were acquired for this study. All chimpanzee MRIs were obtained from a data archive of scans obtained prior to the 2015 implementation of US Fish and Wildlife Service and National Institutes of Health regulations governing research with chimpanzees. All the scans reported in this publication were completed by the end of 2012. All chimpanzee MRI scans are part of the National Chimpanzee Brain Resource (http://www.chimpanzeebrain.org).” Published under the PNAS license. Published online April 29, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1906107116 9680 | PNAS | May 7, 2019 | vol. 116 | no. 19 www.pnas.org Downloaded by guest on October 1, 2021 Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees Dirk Jan Ardescha, Lianne H. Scholtensa, Longchuan Lib, Todd M. Preussc,d,e, James K. Rillingc,d,f,g,h, and Martijn P. van den Heuvela,i,1 aConnectome Lab, Department of Complex Trait Genetics, Center for Neurogenomics and Cognitive Research, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, 1081 HV Amsterdam, The Netherlands; bMarcus Autism Center, Children’s Healthcare of Atlanta, Emory University, Atlanta, GA 30329; cYerkes National Primate Research Center, Emory University, Atlanta, GA 30329; dCenter for Translational Social Neuroscience, Emory University, Atlanta, GA 30329; eDepartment of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA 30307; fDepartment of Anthropology, Emory University, Atlanta, GA 30322; gSilvio O. -

Acute Cognitive Deficits After Traumatic Brain Injury Predict Alzheimer's
GeroScience (2020) 42:1411–1429 https://doi.org/10.1007/s11357-020-00245-6 ORIGINAL ARTICLE Acute cognitive deficits after traumatic brain injury predict Alzheimer’s disease-like degradation of the human default mode network Andrei Irimia & Alexander S. Maher & Nikhil N. Chaudhari & Nahian F. Chowdhury & Elliot B. Jacobs & the Alzheimer’s Disease Neuroimaging Initiative Received: 9 June 2020 /Accepted: 29 July 2020 /Published online: 2 August 2020 # American Aging Association 2020 Abstract Traumatic brain injury (TBI) and Alzheimer’s are leveraged to study the extent to which geriatric mild disease (AD) are prominent neurological conditions whose TBI(mTBI)canleadtoAD-like alteration of resting-state neural and cognitive commonalities are poorly understood. activity in the default mode network (DMN). This network The extent of TBI-related neurophysiological abnormali- is found to contain modules whose extent of AD-like, ties has been hypothesized to reflect AD-like neurodegen- posttraumatic degradation can be accurately prognosticat- eration because TBI can increase vulnerability to AD. ed based on the acute cognitive deficits of geriatric mTBI However, it remains challenging to prognosticate AD risk patients with cerebral microbleeds. Aside from establish- partly because the functional relationship between acute ing a predictive physiological association between geriatric posttraumatic sequelae and chronic AD-like degradation mTBI, cognitive impairment, and AD-like functional deg- remains elusive. Here, functional magnetic resonance im- radation, these findings advance the goal of acutely fore- aging (fMRI), network theory, and machine learning (ML) casting mTBI patients’ chronic deviations from normality along AD-like functional trajectories. The association of Andrei Irimia and Alexander S. Maher contributed equally to this geriatric mTBI with AD-like changes in functional brain work. -

Gliomas of the Cingulate Gyrus: Surgical Management and Functional Outcome
Neurosurg Focus 27 (2):E9, 2009 Gliomas of the cingulate gyrus: surgical management and functional outcome MAREC VON LEHE , M.D., AN D JOHANNES SCHRA mm , M.D. Neurochirurgische Klinik, Universitätsklinik Bonn, Germany Object. In this paper, the authors’ goal was to summarize their experience with the surgical treatment of gliomas arising from the cingulate gyrus. Methods. The authors analyzed preoperative data, surgical strategies, complications, and functional outcome in a series of 34 patients (mean age 42 years, range 12–69 years; 14 females) who underwent 38 operations between May 2001 and November 2008. Results. In 7 cases (18%) the tumor was located in the posterior (parietal) part of the cingulate gyrus, and in 31 (82%) the tumor was in the anterior (frontal) part. In 10 cases (26%) the glioma was solely located in the cingulate gyrus, and in 28 cases (74%) the tumor extended to the supracingular frontal/parietal cortex. Most cases (23 [61%]) had seizures as the presenting symptom, 8 patients (24%) suffered from a hemiparesis/hemihypesthesia, and 4 pa- tients (12%) had aphasic symptoms. The authors chose an interhemispheric approach for tumor resection in 11 (29%) and a transcortical approach in 27 (71%) cases; intraoperative electrophysiological monitoring was applied in 23 (61%) and neuronavigation in 15 (39%) cases. A > 90% resection was achieved in 32 (84%) and > 70% in another 5 (13%) cases. Tumors were classified as low-grade gliomas in 11 cases (29%). A glioblastoma multiforme (WHO Grade IV, 10 cases [26%]) and oligoastrocytoma (WHO Grade III, 9 cases [24%]) were the most frequent histopathological results. -

Patient-Tailored Connectomics Visualization for the Assessment of White Matter Atrophy in Traumatic Brain Injury
Patient-Tailored Connectomics Visualization for the Assessment of White Matter Atrophy in Traumatic Brain Injury The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Irimia, Andrei, Micah C. Chambers, Carinna M. Torgerson, Maria Filippou, David A. Hovda, Jeffry R. Alger, Guido Gerig, et al. 2012. Patient-tailored connectomics visualization for the assessment of white matter atrophy in traumatic brain injury. Frontiers in Neurology 3:10. Published Version doi://10.3389/fneur.2012.00010 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:8462352 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA METHODS ARTICLE published: 06 February 2012 doi: 10.3389/fneur.2012.00010 Patient-tailored connectomics visualization for the assessment of white matter atrophy in traumatic brain injury Andrei Irimia1, Micah C. Chambers 1, Carinna M.Torgerson1, Maria Filippou 2, David A. Hovda2, Jeffry R. Alger 3, Guido Gerig 4, Arthur W.Toga1, Paul M. Vespa2, Ron Kikinis 5 and John D. Van Horn1* 1 Laboratory of Neuro Imaging, Department of Neurology, University of California Los Angeles, Los Angeles, CA, USA 2 Brain Injury Research Center, Departments of Neurology and Neurosurgery, University of California Los Angeles, Los Angeles, CA, USA 3 Department of Radiology, David -

White Matter Dissection and Structural Connectivity of the Human Vertical
www.nature.com/scientificreports OPEN White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex Tatsuya Jitsuishi1, Seiichiro Hirono2, Tatsuya Yamamoto1,3, Keiko Kitajo1, Yasuo Iwadate2 & Atsushi Yamaguchi1* The vertical occipital fasciculus (VOF) is an association fber tract coursing vertically at the posterolateral corner of the brain. It is re-evaluated as a major fber tract to link the dorsal and ventral visual stream. Although previous tractography studies showed the VOF’s cortical projections fall in the dorsal and ventral visual areas, the post-mortem dissection study for the validation remains limited. First, to validate the previous tractography data, we here performed the white matter dissection in post-mortem brains and demonstrated the VOF’s fber bundles coursing between the V3A/B areas and the posterior fusiform gyrus. Secondly, we analyzed the VOF’s structural connectivity with difusion tractography to link vision-associated cortical areas of the HCP MMP1.0 atlas, an updated map of the human cerebral cortex. Based on the criteria the VOF courses laterally to the inferior longitudinal fasciculus (ILF) and craniocaudally at the posterolateral corner of the brain, we reconstructed the VOF’s fber tracts and found the widespread projections to the visual cortex. These fndings could suggest a crucial role of VOF in integrating visual information to link the broad visual cortex as well as in connecting the dual visual stream. Te VOF is the fber tract that courses vertically at the posterolateral corner of the brain. Te VOF was histori- cally described in monkey by Wernicke1 and then in human by Obersteiner2. -

Disconnectome of the Migraine Brain: a “Connectopathy”
Silvestro et al. The Journal of Headache and Pain (2021) 22:102 The Journal of Headache https://doi.org/10.1186/s10194-021-01315-6 and Pain RESEARCH ARTICLE Open Access Disconnectome of the migraine brain: a “connectopathy” model Marcello Silvestro1,2†, Alessandro Tessitore1,2†, Giuseppina Caiazzo1,2, Fabrizio Scotto di Clemente1,2,3, Francesca Trojsi1,2, Mario Cirillo1, Fabrizio Esposito1,2, Gioacchino Tedeschi1,2,3 and Antonio Russo1,2,3* Abstract Background: In the past decades a plethora of studies has been conducted to explore resting-state functional connectivity (RS-FC) of the brain networks in migraine with conflicting results probably due to the variability and susceptibility of signal fluctuations across the course of RS-FC scan. On the other hand, the structural substrates enabling the functional communications among the brain connectome, characterized by higher stability and reproducibility, have not been widely investigated in migraine by means of graph analysis approach. We hypothesize a rearrangement of the brain connectome with an increase of both strength and density of connections between cortical areas specifically involved in pain perception, processing and modulation in migraine patients. Moreover, such connectome rearrangement, inducing an imbalance between the competing parameters of network efficiency and segregation, may underpin a mismatch between energy resources and demand representing the neuronal correlate of the energetically dysfunctional migraine brain. Methods: We investigated, using diffusion-weighted MRI imaging tractography-based graph analysis, the graph- topological indices of the brain “connectome”, a set of grey matter regions (nodes) structurally connected by white matter paths (edges) in 94 patients with migraine without aura compared to 91 healthy controls. -

Brain Mapping Using Neuroimaging
pISSN 2287-5123·eISSN 2287-4445 https://doi.org/10.9729/AM.2016.46.4.179 Review Article Brain Mapping Using Neuroimaging Woo-Suk Tae1, Shin-Hyuk Kang1,2, Byung-Joo Ham1,3, Byung-Jo Kim1,4, Sung-Bom Pyun1,5,* 1Brain Convergence Research Center, Korea University, Seoul 02841, Korea 2Department of Neurosurgery, Korea University College of Medicine, Seoul 02841, Korea 3Department of Psychiatry, Korea University College of Medicine, Seoul 02841, Korea 4Department of Neurology, Korea University College of Medicine, Seoul 02841, Korea 5Department of Physical Medicine and Rehabilitation, Korea University College of Medicine, Seoul 02841, Korea Mapping brain structural and functional connections through the whole brain is essential for understanding brain mechanisms and the physiological bases of brain diseases. Although region specific structural or functional deficits cause brain diseases, the changes of interregional connections could also be important factors of brain diseases. This review will introduce common neuroimaging modalities, including structural magnetic resonance *Correspondence to: imaging (MRI), functional MRI (fMRI), diffusion tensor imaging, and other recent Pyun SB, neuroimaging analyses methods, such as voxel-based morphometry, cortical thickness Tel: +82-2-920-6480 analysis, local gyrification index, and shape analysis for structural imaging. Tract-Based Fax: +82-2-929-9951 Spatial Statistics, TRActs Constrained by UnderLying Anatomy for diffusion MRI, and E-mail: [email protected] independent component analysis for fMRI also will also be introduced. Received December 25, 2016 Revised December 28, 2016 Key Words: Brain connectome, Magnetic resonance imaging, Diffusion tensor imaging, Accepted December 28, 2016 Functional magnetic resonance imaging, Brain mapping INTRODUCTION Hu et al., 2016). -
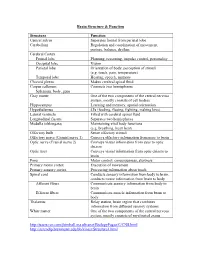
Brain Structure & Function Structure Function Central Sulcus Separates
Brain Structure & Function Structure Function Central sulcus Separates frontal from parietal lobe Cerebellum Regulation and coordination of movement, posture, balance, rhythm Cerebral Cortex Frontal lobe Planning, reasoning, impulse control, personality Occipital lobe Vision Parietal lobe Orientation of body, perception of stimuli (e.g. touch, pain, temperature) Temporal lobe Hearing, speech, memory Choroid plexus Makes cerebral spinal fluid Corpus callosum Connects two hemispheres Splenium, body, genu Gray matter One of the two components of the central nervous system, mostly consists of cell bodies Hippocampus Learning and memory, spatial orientation Hypothalamus 4Fs (feeding, fleeing, fighting, making love) Lateral ventricle Filled with cerebral spinal fluid Longitudinal fissure Separates two hemispheres Medulla (oblongata) Maintaining vital body functions (e.g. breathing, heart beat) Olfactory bulb Sense olfactory stimuli Olfactory nerve (Cranial nerve 1) Conveys olfactory information from nose to brain Optic nerve (Cranial nerve 2) Conveys visual information from eyes to optic chiasm Optic tract Conveys visual information from optic chiasm to brain Pons Motor control, consciousness, alertness Primary motor cortex Execution of movement Primary sensory cortex Processing information about touch Spinal cord Conducts sensory information from body to brain, conducts motor information from brain to body Afferent fibers Communicate sensory information from body to brain Efferent fibers Communicate muscle information from brain to body Thalamus Relay station, brain region that combines information from different sensory systems White matter One of the two components of the central nervous system, mostly consists of myelinated axons http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CNS.html http://serendip.brynmawr.edu/bb/kinser/Structure1.html .