A Landmark for Identification of the Central Sulcus Using Functional Magnetic Resonance Imaging
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Vein Artery
1 PRE-LAB EXERCISES Open the Atlas app. From the Views menu, go to System Views and scroll down to Circulatory System Views. You are responsible for the identification of all bold terms. A. Circulatory System Overview In the Circulatory System Views section, select View 1. Circulatory System. The skeletal system is included in this view. Note that blood vessels travel throughout the entire body. Heart Artery Vein 2 Brachiocephalic trunk Pulmonary circulation Pericardium 1. Where would you find the blood vessels with the largest diameter? 2. Select a few vessels in the leg and read their names. The large blue-colored vessels are _______________________________ and the large red-colored vessels are_______________________________. 3. In the system tray on the left side of the screen, deselect the skeletal system icon to remove the skeletal system structures from the view. The largest arteries and veins are all connected to the _______________________________. 4. Select the heart to highlight the pericardium. Use the Hide button in the content box to hide the pericardium from the view and observe the heart muscle and the vasculature of the heart. 3 a. What is the largest artery that supplies the heart? b. What are the two large, blue-colored veins that enter the right side of the heart? c. What is the large, red-colored artery that exits from the top of the heart? 5. Select any of the purple-colored branching vessels inside the rib cage and use the arrow in the content box to find and choose Pulmonary circulation from the hierarchy list. This will highlight the circulatory route that takes deoxygenated blood to the lungs and returns oxygenated blood back to the heart. -

Pelvic Anatomyanatomy
PelvicPelvic AnatomyAnatomy RobertRobert E.E. Gutman,Gutman, MDMD ObjectivesObjectives UnderstandUnderstand pelvicpelvic anatomyanatomy Organs and structures of the female pelvis Vascular Supply Neurologic supply Pelvic and retroperitoneal contents and spaces Bony structures Connective tissue (fascia, ligaments) Pelvic floor and abdominal musculature DescribeDescribe functionalfunctional anatomyanatomy andand relevantrelevant pathophysiologypathophysiology Pelvic support Urinary continence Fecal continence AbdominalAbdominal WallWall RectusRectus FasciaFascia LayersLayers WhatWhat areare thethe layerslayers ofof thethe rectusrectus fasciafascia AboveAbove thethe arcuatearcuate line?line? BelowBelow thethe arcuatearcuate line?line? MedianMedial umbilicalumbilical fold Lateralligaments umbilical & folds folds BonyBony AnatomyAnatomy andand LigamentsLigaments BonyBony PelvisPelvis TheThe bonybony pelvispelvis isis comprisedcomprised ofof 22 innominateinnominate bones,bones, thethe sacrum,sacrum, andand thethe coccyx.coccyx. WhatWhat 33 piecespieces fusefuse toto makemake thethe InnominateInnominate bone?bone? PubisPubis IschiumIschium IliumIlium ClinicalClinical PelvimetryPelvimetry WhichWhich measurementsmeasurements thatthat cancan bebe mademade onon exam?exam? InletInlet DiagonalDiagonal ConjugateConjugate MidplaneMidplane InterspinousInterspinous diameterdiameter OutletOutlet TransverseTransverse diameterdiameter ((intertuberousintertuberous)) andand APAP diameterdiameter ((symphysissymphysis toto coccyx)coccyx) -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Vascular Density and Distribution in Neocortex
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Vascular density and distribution in neocortex Schmid, Franca ; Barrett, Matthew J P ; Jenny, Patrick ; Weber, Bruno Abstract: An amazingly wide range of complex behavior emerges from the cerebral cortex. Much of the information processing that leads to these behaviors is performed in neocortical circuits that span throughout the six layers of the cortex. Maintaining this circuit activity requires substantial quantities of oxygen and energy substrates, which are delivered by the complex yet well-organized and tightly-regulated vascular system. In this review, we provide a detailed characterization of the most relevant anatomical and functional features of the cortical vasculature. This includes a compilation of the available data on laminar variation of vascular density and the topological aspects of the microvascular system. We also review the spatio-temporal dynamics of cortical blood flow regulation and oxygenation, many aspects of which remain poorly understood. Finally, we discuss some of the important implications of vascular density, distribution, oxygenation and blood flow regulation for (laminar) fMRI. DOI: https://doi.org/10.1016/j.neuroimage.2017.06.046 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-146003 Journal Article Accepted Version The following work is licensed under a Creative Commons: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License. Originally published at: Schmid, Franca; Barrett, Matthew J P; Jenny, Patrick; Weber, Bruno (2019). Vascular density and distribution in neocortex. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -
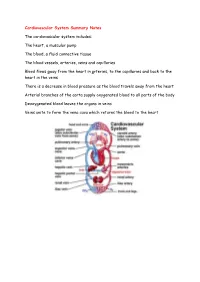
Cardiovascular System Summary Notes the Cardiovascular System
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Gliomas of the Cingulate Gyrus: Surgical Management and Functional Outcome
Neurosurg Focus 27 (2):E9, 2009 Gliomas of the cingulate gyrus: surgical management and functional outcome MAREC VON LEHE , M.D., AN D JOHANNES SCHRA mm , M.D. Neurochirurgische Klinik, Universitätsklinik Bonn, Germany Object. In this paper, the authors’ goal was to summarize their experience with the surgical treatment of gliomas arising from the cingulate gyrus. Methods. The authors analyzed preoperative data, surgical strategies, complications, and functional outcome in a series of 34 patients (mean age 42 years, range 12–69 years; 14 females) who underwent 38 operations between May 2001 and November 2008. Results. In 7 cases (18%) the tumor was located in the posterior (parietal) part of the cingulate gyrus, and in 31 (82%) the tumor was in the anterior (frontal) part. In 10 cases (26%) the glioma was solely located in the cingulate gyrus, and in 28 cases (74%) the tumor extended to the supracingular frontal/parietal cortex. Most cases (23 [61%]) had seizures as the presenting symptom, 8 patients (24%) suffered from a hemiparesis/hemihypesthesia, and 4 pa- tients (12%) had aphasic symptoms. The authors chose an interhemispheric approach for tumor resection in 11 (29%) and a transcortical approach in 27 (71%) cases; intraoperative electrophysiological monitoring was applied in 23 (61%) and neuronavigation in 15 (39%) cases. A > 90% resection was achieved in 32 (84%) and > 70% in another 5 (13%) cases. Tumors were classified as low-grade gliomas in 11 cases (29%). A glioblastoma multiforme (WHO Grade IV, 10 cases [26%]) and oligoastrocytoma (WHO Grade III, 9 cases [24%]) were the most frequent histopathological results. -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -

Lymph and Lymphatic Vessels
Cardiovascular System LYMPH AND LYMPHATIC VESSELS Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Copyright © 2010 Pearson Education, Inc. Figure 19.2 Regional Internal jugular vein lymph nodes: Cervical nodes Entrance of right lymphatic duct into vein Entrance of thoracic duct into vein Axillary nodes Thoracic duct Cisterna chyli Aorta Inguinal nodes Lymphatic collecting vessels Drained by the right lymphatic duct Drained by the thoracic duct (a) General distribution of lymphatic collecting vessels and regional lymph nodes. Figure 20.2a Lymphatic System Outflow of fluid slightly exceeds return Consists of three parts 1. A network of lymphatic vessels carrying lymph 1. Transports fluid back to CV system 2. Lymph nodes 1. Filter the fluid within the vessels 3. Lymphoid organs 1. Participate in disease prevention Lymphatic System Functions 1. Returns interstitial fluid and leaked plasma proteins back to the blood 2. Disease surveillance 3. Lipid transport from intestine via lacteals Venous system Arterial system Heart Lymphatic system: Lymph duct Lymph trunk Lymph node Lymphatic collecting vessels, with valves Tissue fluid Blood Lymphatic capillaries Tissue cell capillary Blood Lymphatic capillaries capillaries (a) Structural relationship between a capillary bed of the blood vascular system and lymphatic capillaries. Filaments anchored to connective tissue Endothelial cell Flaplike minivalve Fibroblast in loose connective tissue (b) Lymphatic capillaries are blind-ended tubes in which adjacent endothelial cells overlap each other, forming flaplike minivalves. -
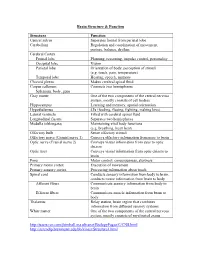
Brain Structure & Function Structure Function Central Sulcus Separates
Brain Structure & Function Structure Function Central sulcus Separates frontal from parietal lobe Cerebellum Regulation and coordination of movement, posture, balance, rhythm Cerebral Cortex Frontal lobe Planning, reasoning, impulse control, personality Occipital lobe Vision Parietal lobe Orientation of body, perception of stimuli (e.g. touch, pain, temperature) Temporal lobe Hearing, speech, memory Choroid plexus Makes cerebral spinal fluid Corpus callosum Connects two hemispheres Splenium, body, genu Gray matter One of the two components of the central nervous system, mostly consists of cell bodies Hippocampus Learning and memory, spatial orientation Hypothalamus 4Fs (feeding, fleeing, fighting, making love) Lateral ventricle Filled with cerebral spinal fluid Longitudinal fissure Separates two hemispheres Medulla (oblongata) Maintaining vital body functions (e.g. breathing, heart beat) Olfactory bulb Sense olfactory stimuli Olfactory nerve (Cranial nerve 1) Conveys olfactory information from nose to brain Optic nerve (Cranial nerve 2) Conveys visual information from eyes to optic chiasm Optic tract Conveys visual information from optic chiasm to brain Pons Motor control, consciousness, alertness Primary motor cortex Execution of movement Primary sensory cortex Processing information about touch Spinal cord Conducts sensory information from body to brain, conducts motor information from brain to body Afferent fibers Communicate sensory information from body to brain Efferent fibers Communicate muscle information from brain to body Thalamus Relay station, brain region that combines information from different sensory systems White matter One of the two components of the central nervous system, mostly consists of myelinated axons http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CNS.html http://serendip.brynmawr.edu/bb/kinser/Structure1.html . -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases.