Vascular Density and Distribution in Neocortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Vein Artery
1 PRE-LAB EXERCISES Open the Atlas app. From the Views menu, go to System Views and scroll down to Circulatory System Views. You are responsible for the identification of all bold terms. A. Circulatory System Overview In the Circulatory System Views section, select View 1. Circulatory System. The skeletal system is included in this view. Note that blood vessels travel throughout the entire body. Heart Artery Vein 2 Brachiocephalic trunk Pulmonary circulation Pericardium 1. Where would you find the blood vessels with the largest diameter? 2. Select a few vessels in the leg and read their names. The large blue-colored vessels are _______________________________ and the large red-colored vessels are_______________________________. 3. In the system tray on the left side of the screen, deselect the skeletal system icon to remove the skeletal system structures from the view. The largest arteries and veins are all connected to the _______________________________. 4. Select the heart to highlight the pericardium. Use the Hide button in the content box to hide the pericardium from the view and observe the heart muscle and the vasculature of the heart. 3 a. What is the largest artery that supplies the heart? b. What are the two large, blue-colored veins that enter the right side of the heart? c. What is the large, red-colored artery that exits from the top of the heart? 5. Select any of the purple-colored branching vessels inside the rib cage and use the arrow in the content box to find and choose Pulmonary circulation from the hierarchy list. This will highlight the circulatory route that takes deoxygenated blood to the lungs and returns oxygenated blood back to the heart. -

Pelvic Anatomyanatomy
PelvicPelvic AnatomyAnatomy RobertRobert E.E. Gutman,Gutman, MDMD ObjectivesObjectives UnderstandUnderstand pelvicpelvic anatomyanatomy Organs and structures of the female pelvis Vascular Supply Neurologic supply Pelvic and retroperitoneal contents and spaces Bony structures Connective tissue (fascia, ligaments) Pelvic floor and abdominal musculature DescribeDescribe functionalfunctional anatomyanatomy andand relevantrelevant pathophysiologypathophysiology Pelvic support Urinary continence Fecal continence AbdominalAbdominal WallWall RectusRectus FasciaFascia LayersLayers WhatWhat areare thethe layerslayers ofof thethe rectusrectus fasciafascia AboveAbove thethe arcuatearcuate line?line? BelowBelow thethe arcuatearcuate line?line? MedianMedial umbilicalumbilical fold Lateralligaments umbilical & folds folds BonyBony AnatomyAnatomy andand LigamentsLigaments BonyBony PelvisPelvis TheThe bonybony pelvispelvis isis comprisedcomprised ofof 22 innominateinnominate bones,bones, thethe sacrum,sacrum, andand thethe coccyx.coccyx. WhatWhat 33 piecespieces fusefuse toto makemake thethe InnominateInnominate bone?bone? PubisPubis IschiumIschium IliumIlium ClinicalClinical PelvimetryPelvimetry WhichWhich measurementsmeasurements thatthat cancan bebe mademade onon exam?exam? InletInlet DiagonalDiagonal ConjugateConjugate MidplaneMidplane InterspinousInterspinous diameterdiameter OutletOutlet TransverseTransverse diameterdiameter ((intertuberousintertuberous)) andand APAP diameterdiameter ((symphysissymphysis toto coccyx)coccyx) -
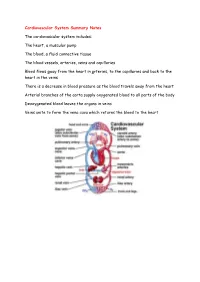
Cardiovascular System Summary Notes the Cardiovascular System
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -

Lymph and Lymphatic Vessels
Cardiovascular System LYMPH AND LYMPHATIC VESSELS Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Copyright © 2010 Pearson Education, Inc. Figure 19.2 Regional Internal jugular vein lymph nodes: Cervical nodes Entrance of right lymphatic duct into vein Entrance of thoracic duct into vein Axillary nodes Thoracic duct Cisterna chyli Aorta Inguinal nodes Lymphatic collecting vessels Drained by the right lymphatic duct Drained by the thoracic duct (a) General distribution of lymphatic collecting vessels and regional lymph nodes. Figure 20.2a Lymphatic System Outflow of fluid slightly exceeds return Consists of three parts 1. A network of lymphatic vessels carrying lymph 1. Transports fluid back to CV system 2. Lymph nodes 1. Filter the fluid within the vessels 3. Lymphoid organs 1. Participate in disease prevention Lymphatic System Functions 1. Returns interstitial fluid and leaked plasma proteins back to the blood 2. Disease surveillance 3. Lipid transport from intestine via lacteals Venous system Arterial system Heart Lymphatic system: Lymph duct Lymph trunk Lymph node Lymphatic collecting vessels, with valves Tissue fluid Blood Lymphatic capillaries Tissue cell capillary Blood Lymphatic capillaries capillaries (a) Structural relationship between a capillary bed of the blood vascular system and lymphatic capillaries. Filaments anchored to connective tissue Endothelial cell Flaplike minivalve Fibroblast in loose connective tissue (b) Lymphatic capillaries are blind-ended tubes in which adjacent endothelial cells overlap each other, forming flaplike minivalves. -
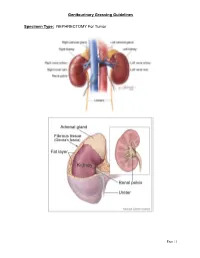
Genitourinary Grossing Guidelines Specimen Type: NEPHRECTOMY
Genitourinary Grossing Guidelines Specimen Type: NEPHRECTOMY For Tumor Page | 1 Genitourinary Grossing Guidelines Procedure: 1. Weigh and measure overall dimensions of specimen. Ink the surface of Gerota’s fascia or perinephric fat, either the entire surface or the surface overlying a palpable tumor. 2. Inspect perinephric fat for adrenal, tumor extension; palpate for hilar lymph nodes. 3. Locate ureter (often with a staple or clip at the distal end). Measure the length and diameter of ureter. Locate renal arteries and vein, look for tumor thrombus in the renal vein. 4. Remove vascular and ureteral margins and place in cassette, en face. 5. Place a probe into ureter, and extend it into renal pelvis. Open ureter along its length. Examine ureteral mucosa. 6. At renal hilum, push one probe through renal pelvicalyceal system and push through parenchyma of superior pole of kidney. 7. Place second probe in renal pelvicalyceal system and push through parenchyma of inferior pole of kidney. 8. Using 2 probes as guides, divide kidney in two complete halves, cutting through the renal pelvis. Completely open pelvis, calyces, and renal veins. 9. Measure kidney. 10. Look for renal sinus invasion, renal vein invasion, and capsular invasion by tumor. Measure and describe adrenal (? tumor involvement) if present. 11. Photograph half of the specimen or both halves. 12. Describe tumor: single/multiple lesions, dimensions, demarcation, color, texture, hemorrhage/necrosis/cystic degeneration, extension into renal sinus/into renal vein/through capsular surface, areas of sarcomatoid differentiation. 13. After taking sections of tumor, bread loaf the uninvolved kidney in 1 cm interval, look for additional lesions, describe uninvolved kidney: external surface, cortex, medulla, and pelvis. -

Structural and Mechanical Characterisation of Bridging Veins: a Review
Structural and mechanical characterisation of bridging veins: a review Nele Famaeya,∗, Zhao Ying Cuia,∗, Grace Umuhire Musigazib, Jan Ivensc, Bart Depreitereb, Erik Verbekend, Jos Vander Slotena aBiomechanics Section, KU Leuven, Belgium bDepartment of Neurosurgery, University Hospital Gasthuisberg, KU Leuven, Belgium cComposite Materials Group, Department of Metallurgy and Materials Engineering, KU Leuven, Belgium dTranslational Cell & Tissue Research, KU Leuven, Belgium Abstract Bridging veins drain the venous blood from the cerebral cortex into the supe- rior sagittal sinus (SSS) and doing so they bridge the subdural space. Despite their importance in head impact biomechanics, little is known about their properties with respect to histology, morphology and mechanical behaviour. Knowledge of these characteristics is essential for creating a biofidelic finite element model to study the biomechanics of head impact, ultimately leading to the improved design of protective devices by setting up tolerance criteria. This paper presents a comprehensive review of the state-of-the-art knowledge on bridging veins. Tolerance criteria to prevent head injury through impact have been set by a number of research groups, either directly through impact experiments or by means of finite element (FE) simulations. Current state-of-the-art FE head models still lack a biofidelic representation of the bridging veins. To achieve this, a thorough insight into their nature and behaviour is re- quired. Therefore, an overview of the general morphology and histology is provided here, showing the clearly heterogeneous nature of the bridging vein complex, with its three different layers and distinct morphological and histo- logical changes at the region of outflow into the superior sagittal sinus. -

Biology C - Circulation
Unit: Biology C - Circulation LESSON 1.1 - AN INTRODUCTION TO THE CIRCULATORY SYSTEM Overview: Students will read about the circulatory system and answer probing questions to test their understanding. Suggested Timeline: 1.5 hours Materials: An Introduction to the Circulatory System (Student Handout) Teacher access to computer, projector and the Internet QUIZ – An Introduction to the Circulatory System (Student Handout) Method: INDIVIDUAL FORMAT: 1. Have students complete their vocabulary list, reading and questions on ‘An Introduction to the Circulatory System’ (Student Handout). 2. Set a date for the quiz on the material (‘QUIZ – An Introduction to the Circulatory System’ – Student Handout). GROUP FORMAT: 1. Introduce the circulatory system by showing students the following online video clip: http://pennhealth.com/health_info/animationplayer/cardiac_system.html 2. Have students complete their vocabulary list, reading and questions on ‘An introduction to the Circulatory System’ (Student Handout). 3. Set a date for the quiz on the material (‘QUIZ – An Introduction to the Circulatory System’ – Student Handout). Assessment and Evaluation: Assessment of student’s understanding of material through review of questions Student grade on quiz Science 21 Bio C - Circulation B46 Unit: Biology C - Circulation Student Handout AN INTRODUCTION TO THE CIRCULATORY SYSTEM VOCABULARY blood vessel - arteries – veins – capillaries – blood – plasma – white blood cells – red blood cells – platelets – WHAT IS CIRCULATION? What messenger makes trillions of stops in just thirty seconds? Your blood! In just under thirty seconds, your blood moves (circulates) through your entire body. It reaches every one of your trillions of cells. Blood carries things to each cell that are needed. These things include oxygen and nutrients. -

Right Circumcaval Ureter and Double Right Renal Vein in the Brazilian Shorthair Cat (Felis Catus): Two Case Reports M
Folia Morphol. Vol. 78, No. 2, pp. 433–436 DOI: 10.5603/FM.a2018.0082 C A S E R E P O R T Copyright © 2019 Via Medica ISSN 0015–5659 journals.viamedica.pl Right circumcaval ureter and double right renal vein in the Brazilian shorthair cat (Felis catus): two case reports M. Abidu-Figueiredo1, A.V. Stocco1, C.A. Santos-Sousa1, 2, P. Souza Junior3, L.A.S. Pires4, M.A. Babinski4 1Universidade Federal Rural do Rio de Janeiro, Brazil 2Universidade Federal do Acre, Brazil 3Universidade Federal do Pampa, Brazil 4Universidade Federal Fluminense, Brazil, Brazil [Received: 29 June 2018; Accepted: 19 August 2018] Variations of the renal veins are well described in the literature, although variations concerning the ureter are considered a rare finding in cats. The circumcaval ureter is one of the rarest variations of the ureter and is characterised by a loop of the ureter posterior to the caudal vena cava. This variant is also known as preureteral vena cava and retrocaval ureter. It is thought to be caused by a deviation during embryonic development of the aforementioned vein. Due to its rarity, there are scarce reports of the circumcaval ureter in cats, and its association with two renal veins makes it less common as well. These variations should be preoperatively identified in order to avoid complications in kidney transplants, ureteral surgeries and cystoscopies, for instance. The present work aims to report two cases of a circumcaval ureter with two renal veins in two different Brazilian shorthair cats (Felis catus). (Folia Morphol 2019; 78, 2: 433–436) Key words: anatomical variations, circumcaval ureter, preureteral vena cava, retrocaval ureter, cat, renal vein INTRODUCTION pedicle and is essential to avoid confusion during The renal pedicle is composed by the ureter, one imaging exams [4, 8]. -

The Digestive System
THE DIGESTIVE SYSTEM COMPILED BY HOWIE BAUM DIGESTIVE SYSTEM People are probably more aware of their digestive system than of any other system, not least because of its frequent messages. Hunger, thirst, appetite, gas ☺, and the frequency and nature of bowel movements, are all issues affecting daily life. The Digestive Tract • Six Functions of the Digestive System 1. Ingestion 2. Mechanical processing 3. Digestion 4. Secretion 5. Absorption 6. Excretion The Digestive Tract • Ingestion – Occurs when materials enter digestive tract via the mouth • Mechanical Processing – Crushing and shearing – Makes materials easier to propel along digestive tract • Digestion – The chemical breakdown of food into small organic fragments for absorption by digestive epithelium The Digestive Tract • Secretion – Is the release of water, acids, enzymes, buffers, and salts – By epithelium of digestive tract – By glandular organs • Absorption – Movement of organic substrates, electrolytes, vitamins, and water – Across digestive epithelium tissue – Into the interstitial fluid of digestive tract • Excretion – Removal of waste products from body fluids – Process called defecation removes feces AN INTRODUCTION TO THE DIGESTIVE SYSTEM • The Digestive Tract • Also called the gastrointestinal (GI) tract or alimentary canal • Is a muscular tube • Extends from our mouth to the anus • Passes through the pharynx, esophagus, stomach, and small and large intestines The digestive system is one of the most clearly defined in the body. It consists of a long passageway, the digestive -

Anatomy and Physiology of the Cardiovascular System
Chapter © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 5 NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Anatomy© Jonesand & Physiology Bartlett Learning, LLC of © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION the Cardiovascular System © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION OUTLINE Aortic arch: The second section of the aorta; it branches into Introduction the brachiocephalic trunk, left common carotid artery, and The Heart left subclavian artery. Structures of the Heart Aortic valve: Located at the base of the aorta, the aortic Conduction System© Jones & Bartlett Learning, LLCvalve has three cusps and opens© Jonesto allow blood & Bartlett to leave the Learning, LLC Functions of the HeartNOT FOR SALE OR DISTRIBUTIONleft ventricle during contraction.NOT FOR SALE OR DISTRIBUTION The Blood Vessels and Circulation Arteries: Elastic vessels able to carry blood away from the Blood Vessels heart under high pressure. Blood Pressure Arterioles: Subdivisions of arteries; they are thinner and have Blood Circulation muscles that are innervated by the sympathetic nervous Summary© Jones & Bartlett Learning, LLC system. © Jones & Bartlett Learning, LLC Atria: The upper chambers of the heart; they receive blood CriticalNOT Thinking FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Websites returning to the heart. Review Questions Atrioventricular node (AV node): A mass of specialized tissue located in the inferior interatrial septum beneath OBJECTIVES the endocardium; it provides the only normal conduction pathway between the atrial and ventricular syncytia. -

Blood Vessels and Circulation
C h a p t e r 13 Blood Vessels and Circulation PowerPoint® Lecture Slides prepared by Jason LaPres Lone Star College - North Harris Copyright © 2010 Pearson Education, Inc. Copyright © 2010 Pearson Education, Inc. 13-1 Arteries, arterioles, capillaries, venules, and veins differ in size, structure, and function Copyright © 2010 Pearson Education, Inc. Classes of Blood Vessels • Arteries – Carry blood away from the heart • Arterioles – Are the smallest branches of arteries • Capillaries – Are the smallest blood vessels – Location of exchange between blood and interstitial fluid • Venules – Collect blood from capillaries • Veins – Return blood to heart Copyright © 2010 Pearson Education, Inc. The Structure of Vessel Walls • Tunica Intima – Innermost endothelial lining and connective tissue • Tunica Media – Is the middle layer – Contains concentric sheets of smooth muscle in loose connective tissue • Tunica Externa – Contains connective tissue sheath Copyright © 2010 Pearson Education, Inc. Typical Artery and a Typical Vein Figure 13-1 Copyright © 2010 Pearson Education, Inc. Arteries • From heart to capillaries, arteries change – From elastic arteries – To muscular arteries – To arterioles Copyright © 2010 Pearson Education, Inc. Arteries • Elastic Arteries – Also called conducting arteries – Large vessels (e.g., pulmonary trunk and aorta) – Tunica media has many elastic fibers and few muscle cells – Elasticity evens out pulse force Copyright © 2010 Pearson Education, Inc. Arteries • Muscular Arteries – Also called distribution arteries – Are medium sized (most arteries) – Tunica media has many muscle cells Copyright © 2010 Pearson Education, Inc. Arteries • Arterioles – Are small – Have little or no tunica externa – Have thin or incomplete tunica media Copyright © 2010 Pearson Education, Inc. Blood Vessels Figure 13-2 Copyright © 2010 Pearson Education, Inc.