Structural and Mechanical Characterisation of Bridging Veins: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Why Do Bridging Veins Rupture Into the Virtual Subdural Space?
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.47.2.121 on 1 February 1984. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1984;47:121-127 Why do bridging veins rupture into the virtual subdural space? T YAMASHIMA, RL FRIEDE From the Department ofNeuropathology, University of Gottingen, Gottingen, Federal Republic of Germany SUMMARY Electron microscopic data on human bridging veins show thin walls of variable thick- ness, circumferential arrangement of collagen fibres and a lack of outer reinforcement by arach- noid trabecules, all contributory to the subdural portion of the vein being more fragile than its subarachnoid portion. These features explain the laceration of veins and the subdural location of resultant haematomas. Most subdural haematomas due to venous bleeding walls are delicate, lacking muscle fibres, with only a have been attributed to lacerations in bridging veins. thin fibrous wall and a thin elastic lamina adjacent to These veins form short trunks passing directly from the endothelial layer. The conclusions of these two the brain to the dura mater, almost at right angles to authors, have gained wide acceptance, although guest. Protected by copyright. both. Between these two points, bridging veins take there was little evidence concerning the fragility of a straight course with no tortuosity to allow for the the vein walls. possible displacement of brain.' Trotter2 speculated The purpose of the present communication is to that subdural haematomas are invariably due to provide electron microscopic data on tissue fixed in trauma tearing large veins, an interpretation situ, which might throw some light on to the lacera- elaborated by Krauland.3 According to Leary,4 the tion mechanism of bridging veins and its relationship common sources of subdural haematomas are rup- to the development of subdural haematoma. -

Heart Vein Artery
1 PRE-LAB EXERCISES Open the Atlas app. From the Views menu, go to System Views and scroll down to Circulatory System Views. You are responsible for the identification of all bold terms. A. Circulatory System Overview In the Circulatory System Views section, select View 1. Circulatory System. The skeletal system is included in this view. Note that blood vessels travel throughout the entire body. Heart Artery Vein 2 Brachiocephalic trunk Pulmonary circulation Pericardium 1. Where would you find the blood vessels with the largest diameter? 2. Select a few vessels in the leg and read their names. The large blue-colored vessels are _______________________________ and the large red-colored vessels are_______________________________. 3. In the system tray on the left side of the screen, deselect the skeletal system icon to remove the skeletal system structures from the view. The largest arteries and veins are all connected to the _______________________________. 4. Select the heart to highlight the pericardium. Use the Hide button in the content box to hide the pericardium from the view and observe the heart muscle and the vasculature of the heart. 3 a. What is the largest artery that supplies the heart? b. What are the two large, blue-colored veins that enter the right side of the heart? c. What is the large, red-colored artery that exits from the top of the heart? 5. Select any of the purple-colored branching vessels inside the rib cage and use the arrow in the content box to find and choose Pulmonary circulation from the hierarchy list. This will highlight the circulatory route that takes deoxygenated blood to the lungs and returns oxygenated blood back to the heart. -

Pelvic Anatomyanatomy
PelvicPelvic AnatomyAnatomy RobertRobert E.E. Gutman,Gutman, MDMD ObjectivesObjectives UnderstandUnderstand pelvicpelvic anatomyanatomy Organs and structures of the female pelvis Vascular Supply Neurologic supply Pelvic and retroperitoneal contents and spaces Bony structures Connective tissue (fascia, ligaments) Pelvic floor and abdominal musculature DescribeDescribe functionalfunctional anatomyanatomy andand relevantrelevant pathophysiologypathophysiology Pelvic support Urinary continence Fecal continence AbdominalAbdominal WallWall RectusRectus FasciaFascia LayersLayers WhatWhat areare thethe layerslayers ofof thethe rectusrectus fasciafascia AboveAbove thethe arcuatearcuate line?line? BelowBelow thethe arcuatearcuate line?line? MedianMedial umbilicalumbilical fold Lateralligaments umbilical & folds folds BonyBony AnatomyAnatomy andand LigamentsLigaments BonyBony PelvisPelvis TheThe bonybony pelvispelvis isis comprisedcomprised ofof 22 innominateinnominate bones,bones, thethe sacrum,sacrum, andand thethe coccyx.coccyx. WhatWhat 33 piecespieces fusefuse toto makemake thethe InnominateInnominate bone?bone? PubisPubis IschiumIschium IliumIlium ClinicalClinical PelvimetryPelvimetry WhichWhich measurementsmeasurements thatthat cancan bebe mademade onon exam?exam? InletInlet DiagonalDiagonal ConjugateConjugate MidplaneMidplane InterspinousInterspinous diameterdiameter OutletOutlet TransverseTransverse diameterdiameter ((intertuberousintertuberous)) andand APAP diameterdiameter ((symphysissymphysis toto coccyx)coccyx) -

Gross Anatomy
www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY www.BookOfLinks.com Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The authors and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the possibility of human error or changes in medical sciences, neither the authors nor the publisher nor any other party who has been involved in the preparation or publication of this work warrants that the information contained herein is in every respect accurate or complete, and they disclaim all responsibility for any errors or omissions or for the results obtained from use of the information contained in this work. Readers are encouraged to confirm the infor- mation contained herein with other sources. For example and in particular, readers are advised to check the product information sheet included in the package of each drug they plan to administer to be certain that the information contained in this work is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infrequently used drugs. www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY David A. Morton, PhD Associate Professor Anatomy Director Department of Neurobiology and Anatomy University of Utah School of Medicine Salt Lake City, Utah K. Bo Foreman, PhD, PT Assistant Professor Anatomy Director University of Utah College of Health Salt Lake City, Utah Kurt H. -

Vascular Density and Distribution in Neocortex
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Vascular density and distribution in neocortex Schmid, Franca ; Barrett, Matthew J P ; Jenny, Patrick ; Weber, Bruno Abstract: An amazingly wide range of complex behavior emerges from the cerebral cortex. Much of the information processing that leads to these behaviors is performed in neocortical circuits that span throughout the six layers of the cortex. Maintaining this circuit activity requires substantial quantities of oxygen and energy substrates, which are delivered by the complex yet well-organized and tightly-regulated vascular system. In this review, we provide a detailed characterization of the most relevant anatomical and functional features of the cortical vasculature. This includes a compilation of the available data on laminar variation of vascular density and the topological aspects of the microvascular system. We also review the spatio-temporal dynamics of cortical blood flow regulation and oxygenation, many aspects of which remain poorly understood. Finally, we discuss some of the important implications of vascular density, distribution, oxygenation and blood flow regulation for (laminar) fMRI. DOI: https://doi.org/10.1016/j.neuroimage.2017.06.046 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-146003 Journal Article Accepted Version The following work is licensed under a Creative Commons: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License. Originally published at: Schmid, Franca; Barrett, Matthew J P; Jenny, Patrick; Weber, Bruno (2019). Vascular density and distribution in neocortex. -

Dynamic Assessment of Venous Anatomy and Function in Neurosurgery with Real-Time Intraoperative Multimodal Ultrasound: Technical Note
NEUROSURGICAL FOCUS Neurosurg Focus 45 (1):E6, 2018 Dynamic assessment of venous anatomy and function in neurosurgery with real-time intraoperative multimodal ultrasound: technical note Francesco Prada, MD,1,2 Massimiliano Del Bene, MD,1,3 Giovanni Mauri, MD,4 Massimo Lamperti, MD,5 Davide Vailati, MD,6 Carla Richetta, MD,7 Marco Saini, MD,1 Davide Santuari, MD,8 M. Yashar S. Kalani, MD, PhD,2 and Francesco DiMeco, MD1,9 1Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico C. Besta, Milan, Italy; 2Department of Neurological Surgery, University of Virginia Health Science Center, Charlottesville, Virginia; Departments of 3Experimental Oncology and 4Radiology, European Institute of Oncology, Milan, Italy; 5Anesthesiology Unit, Cleveland Clinic, Abu Dhabi, United Arab Emirates; 6Anesthesiology Unit, Ospedale di Circolo di Melegnano, Presidio di Vizzolo Predabissi, Milan, Italy; 7Department of Neurosurgery, Sourasky Medical Center, Tel Aviv, Israel; 8Department of Vascular Surgery, Ospedale S. Carlo, Milan, Italy; and 9Department of Neurological Surgery, Johns Hopkins Medical School, Baltimore, Maryland The relevance of the cerebral venous system is often underestimated during neurosurgical procedures. Damage to this draining system can have catastrophic implications for the patient. Surgical decision-making and planning must consider each component of the venous compartment, from the medullary draining vein to the dural sinuses and extracranial veins. Intraoperative ultrasound (ioUS) permits the real-time study of venous compartments using different modalities, thus allowing complete characterization of their anatomical and functional features. The B-mode (brightness mode) offers a high-resolution anatomical representation of veins and their relationships with lesions. Doppler modalities (color, power, spectral) allow the study of blood flow and identification of vessels to distinguish their functional characteristics. -
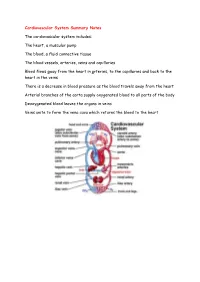
Cardiovascular System Summary Notes the Cardiovascular System
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Dural Venous Channels: Hidden in Plain Sight–Reassessment of an Under-Recognized Entity
Published July 16, 2020 as 10.3174/ajnr.A6647 ORIGINAL RESEARCH INTERVENTIONAL Dural Venous Channels: Hidden in Plain Sight–Reassessment of an Under-Recognized Entity M. Shapiro, K. Srivatanakul, E. Raz, M. Litao, E. Nossek, and P.K. Nelson ABSTRACT BACKGROUND AND PURPOSE: Tentorial sinus venous channels within the tentorium cerebelli connecting various cerebellar and su- pratentorial veins, as well as the basal vein, to adjacent venous sinuses are a well-recognized entity. Also well-known are “dural lakes” at the vertex. However, the presence of similar channels in the supratentorial dura, serving as recipients of the Labbe, super- ficial temporal, and lateral and medial parieto-occipital veins, among others, appears to be underappreciated. Also under-recog- nized is the possible role of these channels in the angioarchitecture of certain high-grade dural fistulas. MATERIALS AND METHODS: A retrospective review of 100 consecutive angiographic studies was performed following identification of index cases to gather data on the angiographic and cross-sectional appearance, location, length, and other features. A review of 100 consecutive dural fistulas was also performed to identify those not directly involving a venous sinus. RESULTS: Supratentorial dural venous channels were found in 26% of angiograms. They have the same appearance as those in the tentorium cerebelli, a flattened, ovalized morphology owing to their course between 2 layers of the dura, in contradistinction to a rounded cross-section of cortical and bridging veins. They are best appreciated on angiography and volumetric postcontrast T1- weighted images. Ten dural fistulas not directly involving a venous sinus were identified, 6 tentorium cerebelli and 4 supratentorial. -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -

Lymph and Lymphatic Vessels
Cardiovascular System LYMPH AND LYMPHATIC VESSELS Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Copyright © 2010 Pearson Education, Inc. Figure 19.2 Regional Internal jugular vein lymph nodes: Cervical nodes Entrance of right lymphatic duct into vein Entrance of thoracic duct into vein Axillary nodes Thoracic duct Cisterna chyli Aorta Inguinal nodes Lymphatic collecting vessels Drained by the right lymphatic duct Drained by the thoracic duct (a) General distribution of lymphatic collecting vessels and regional lymph nodes. Figure 20.2a Lymphatic System Outflow of fluid slightly exceeds return Consists of three parts 1. A network of lymphatic vessels carrying lymph 1. Transports fluid back to CV system 2. Lymph nodes 1. Filter the fluid within the vessels 3. Lymphoid organs 1. Participate in disease prevention Lymphatic System Functions 1. Returns interstitial fluid and leaked plasma proteins back to the blood 2. Disease surveillance 3. Lipid transport from intestine via lacteals Venous system Arterial system Heart Lymphatic system: Lymph duct Lymph trunk Lymph node Lymphatic collecting vessels, with valves Tissue fluid Blood Lymphatic capillaries Tissue cell capillary Blood Lymphatic capillaries capillaries (a) Structural relationship between a capillary bed of the blood vascular system and lymphatic capillaries. Filaments anchored to connective tissue Endothelial cell Flaplike minivalve Fibroblast in loose connective tissue (b) Lymphatic capillaries are blind-ended tubes in which adjacent endothelial cells overlap each other, forming flaplike minivalves. -

Anatomic Comparison of Veins of Labbé Between Autopsy, Digital Subtraction Angiography and Computed Tomographic Venography
Fang et al. BioMed Eng OnLine (2017) 16:84 DOI 10.1186/s12938-017-0374-3 BioMedical Engineering OnLine RESEARCH Open Access Anatomic comparison of veins of Labbé between autopsy, digital subtraction angiography and computed tomographic venography Qiong Fang1, Anhong Jiang2, Wei Tao3* and Lin Xin4 *Correspondence: [email protected] Abstract 3 Department of Anatomy, Objective: The drainage portion of the vein of Labbé varies, and it is difcult to pre- School of Medicine, Anhui University of Science & dict whether the operation is likely to damage this vein. The aim of this study was to Technology, 25 Dongshan correlate the microanatomy of the vein of Labbé with digital subtraction angiography Road, Huainan 232001, China (DSA) and computed tomographic venography (CTV), in order to provide a basis for Full list of author information is available at the end of the the preservation of the vein of Labbé during a supratentorial surgical approach. article Methods: A total of 30 human cadavers (60 sides) and 61 living patients (110 sides) were examined in this study. Each cadaver head was injected with blue latex via the superior sagittal sinus and the internal jugular veins. The venograms of each patient were obtained from the venous phases of DSA (60 sides for 36 patients) or CTV (50 sides for 25 patients). Results: The patients were divided into four subgroups based on the location where a vein entered the dural sinus: the transverse sinus group, the tentorial group, the petrosal group, and the upper-transverse sinus group. The veins of Labbé in transverse sinus group and petrosal group directly entered dural sinus. -
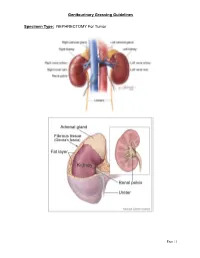
Genitourinary Grossing Guidelines Specimen Type: NEPHRECTOMY
Genitourinary Grossing Guidelines Specimen Type: NEPHRECTOMY For Tumor Page | 1 Genitourinary Grossing Guidelines Procedure: 1. Weigh and measure overall dimensions of specimen. Ink the surface of Gerota’s fascia or perinephric fat, either the entire surface or the surface overlying a palpable tumor. 2. Inspect perinephric fat for adrenal, tumor extension; palpate for hilar lymph nodes. 3. Locate ureter (often with a staple or clip at the distal end). Measure the length and diameter of ureter. Locate renal arteries and vein, look for tumor thrombus in the renal vein. 4. Remove vascular and ureteral margins and place in cassette, en face. 5. Place a probe into ureter, and extend it into renal pelvis. Open ureter along its length. Examine ureteral mucosa. 6. At renal hilum, push one probe through renal pelvicalyceal system and push through parenchyma of superior pole of kidney. 7. Place second probe in renal pelvicalyceal system and push through parenchyma of inferior pole of kidney. 8. Using 2 probes as guides, divide kidney in two complete halves, cutting through the renal pelvis. Completely open pelvis, calyces, and renal veins. 9. Measure kidney. 10. Look for renal sinus invasion, renal vein invasion, and capsular invasion by tumor. Measure and describe adrenal (? tumor involvement) if present. 11. Photograph half of the specimen or both halves. 12. Describe tumor: single/multiple lesions, dimensions, demarcation, color, texture, hemorrhage/necrosis/cystic degeneration, extension into renal sinus/into renal vein/through capsular surface, areas of sarcomatoid differentiation. 13. After taking sections of tumor, bread loaf the uninvolved kidney in 1 cm interval, look for additional lesions, describe uninvolved kidney: external surface, cortex, medulla, and pelvis.