Effect of Salinity on the Antiparasitic Activity of Hyssop Essential Oil 2 3 Nesrine Ben Hamidaa, Rafael A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Antibacterial and Antioxidant Effects of Clove (Syzygium Aromaticum) and Lemon Verbena (Aloysia Citriodora) Essential Oils
Journal of Human, Environment, and Health Promotion. 2019; 5(2): 86-93 Journal of Human, Environment, and Health Promotion Journal homepage: www.zums.ac.ir/jhehp The Antibacterial and Antioxidant Effects of Clove (Syzygium aromaticum) and Lemon Verbena (Aloysia citriodora) Essential Oils a b * c d Mahzad Hosseini Abdollah Jamshidi Mojtaba Raeisi Mohammad Azizzadeh a Student of Food Hygiene, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. b Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. c Department of Nutrition, Faculty of Health, Golestan University of Medical sciences, Gorgan, Iran. d Department of Clinical Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. *Corresponding author: Abdollah Jamshidi Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. Postal code: 9187195786. E-mail address: [email protected] ARTICLE INFO ABSTARACT Article type: Background: The study aimed to investigate the chemical composition, antimicrobial Original article effects, and antioxidant properties of clove and lemon verbena essential oils (EOs). Article history: Methods: The chemical composition of the EOs was identified using gas Received 31 March 2019 chromatography/mass spectrometry (GC/MS). In addition, the antibacterial effects of EOs Revised 9 June 2019 against seven important foodborne bacteria were assessed using the disk-diffusion, agar Accepted 20 June 2019 well-diffusion, and broth microdilution assays. Evaluation of the antioxidant properties of the EOs was carried out using DPPH, β-carotene-linoleic acid bleaching, and reducing DOI: 10.29252/jhehp.5.2.7 power assay. Keywords: Results: All the tested bacteria demonstrated susceptibility to EOs, with the highest Essential oil susceptibility observed in Bacillus cereus to the clove EO in the agar disk-diffusion test. -

Research Paper the Effect of Salinity Stress and Temperature Levels
Academia Journal of Medicinal Plants 9(6): 070-077, June 2021 DOI: 10.15413/ajmp.2021.0107 ISSN: 2315-7720 ©2021 Academia Publishing Research Paper The effect of salinity stress and temperature levels on germination characteristics of four medicinal plants seed Accepted 9th March, 2021 ABSTRACT Cultivation of medicinal plants has been economically beneficial for pharmacy and medicine. These plants are stores of active and valuable secondary metabolites that can be converted into various drugs, some of which are life-saving. However, it is difficult to start a large-scale and commercial cultivation of these plants because most of the arable land is used to produce strategically essential crops. Other uncultivable lands are often affected by various abiotic stresses, one of the most important of which is salinity. Germination of plants is one of the important stages during their growth period that is often affected by environmental stresses, especially salinity. In this study, the seeds of medicinal plants such as Lavender (Lavandula angustifolia), hyssop (Hyssopus officinalis), black cumin (Nigella sativa L.) and Scrophularia (Scrophularia striata) were subjected to salinity stress at 20, 25 and 30°C in order to determine the germination characteristics of their seeds. The results of this experiment generally showed that with increasing salinity stress at different temperatures, all germination characteristics including germination percentage, germination rate, shoot length, root length, shoot dry weight, root dry weight and seed vigor index decreased. It seemed that in order to Yousef Hakimi grow plants commercially, low salinity soil and water are needed to get the best yield. Department of Horticulture, Faculty of Agriculture and Natural Resources, University Key words: Medicinal plants, salt stress, seed treatment, germination, lavender of Tehran, Karaj, Iran. -
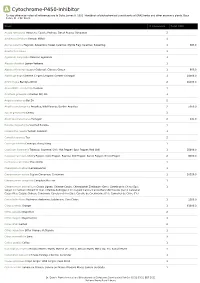
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Industrial Crops and Products 77 (2015) 972–982
Industrial Crops and Products 77 (2015) 972–982 Contents lists available at ScienceDirect Industrial Crops and Products journal homepage: www.elsevier.com/locate/indcrop Extending the use of irradiation to preserve chemical and bioactive properties of medicinal and aromatic plants: A case study with four species submitted to electron beam a a b a Eliana Pereira , Amilcar L. Antonio , Andrzej Rafalski , João C.M. Barreira , a a,∗ Lillian Barros , Isabel C.F.R. Ferreira a Centro de Investigac¸ ão de Montanha (CIMO), ESA, Instituto Politécnico de Braganc¸ a, Campus de Santa Apolónia, 1172, 5301-855 Braganc¸ a, Portugal b Centre for Radiation Research and Technology, Institute of Nuclear Chemistry and Technology, Dorodna Str. 16, 03-195 Warsaw, Poland a r t i c l e i n f o a b s t r a c t Article history: The effects of gamma irradiation on Aloysia citrodora, Melissa officinalis, Melittis melissophyllum and Mentha Received 28 June 2015 piperita were previously evaluated. Herein, the same species were treated with electron-beam irradia- Received in revised form tion (EB) and the same parameters were evaluated. Instead of presenting absolute values for each studied 24 September 2015 parameter, data were evaluated as percentage of induced variation. Besides the newly obtained results, Accepted 29 September 2015 data from a previous work was recalled and normalized in the same manner. Several examples of per- centage variations specific to a plant species or irradiation condition were found. Nevertheless, it was not Keywords: possible to identify unequivocal trends. Even so, when evaluated in an integrative way, the parameters Irradiation with highest discriminating ability among irradiation conditions or plant species were fatty acids and Aromatic plants bioactive indicators. -

Hyssop Herb Yield and Quality Depending on Harvest Term and Plant Spacing
Acta Sci. Pol., Hortorum Cultus 10(3) 2011, 331-342 HYSSOP HERB YIELD AND QUALITY DEPENDING ON HARVEST TERM AND PLANT SPACING GraĪyna ZawiĞlak University of Life Sciences in Lublin Abstract. Hyssop (Hyssopus officinalis L.) is an oil plant, acts antiseptically and stimu- lates digestion. It is applied both for curative and culinary purposes. Studies conducted in the years 2006–2008 were aimed at the effect of plant harvest term (mid June – plants in vegetative phase, mid July – beginning of flowering, mid August – full blooming, mid September – after flowering) and plant spacing (30 × 30, 40 × 40, 50 × 50 cm) upon yielding and quantity of hyssop herb. Studies were conducted at one-year plantation es- tablished from seedlings. Yield of fresh, dry herb and yield of herb without stems was significantly dependent upon the examined factors. Significantly greater fresh herb yield was obtained from plants after flowering (on average: 2.32 kg.m-2), just like the dry yield and yield of herb without stems. In the analysis of the effect of plant spacing upon hyssop yielding, it was revealed that the highest fresh herb yield (on average 1.47 kg.m-2) was from plants grown in the spacing of 40 × 40 cm, similarly to yield of the dry herb and yield of herb without stems. No significant effect of plant spacing was found on the con- tents of dry matter, L-ascorbic acid, chlorophyll, carotenoids, oil, tannins and flavonoids. It was demonstrated, however, that the harvest term significantly effects the contents of L-ascorbic acid, chlorophyll, carotenoids and essential oil in hyssop herb. -

Indiana Medical History Museum Guide to the Medicinal Plant Garden
Indiana Medical History Museum Guide to the Medicinal Plant Garden Garden created and maintained by Purdue Master Gardeners of Marion County IMHM Medicinal Plant Garden Plant List – Common Names Trees and Shrubs: Arborvitae, Thuja occidentalis Culver’s root, Veronicastrum virginicum Black haw, Viburnum prunifolium Day lily, Hemerocallis species Catalpa, Catalpa bignonioides Dill, Anethum graveolens Chaste tree, Vitex agnus-castus Elderberry, Sambucus nigra Dogwood, Cornus florida Elecampane, Inula helenium Elderberry, Sambucus nigra European meadowsweet, Queen of the meadow, Ginkgo, Ginkgo biloba Filipendula ulmaria Hawthorn, Crateagus oxycantha Evening primrose, Oenothera biennis Juniper, Juniperus communis False Solomon’s seal, Smilacina racemosa Redbud, Cercis canadensis Fennel, Foeniculum vulgare Sassafras, Sassafras albidum Feverfew, Tanacetum parthenium Spicebush, Lindera benzoin Flax, Linum usitatissimum Witch hazel, Hamamelis virginiana Foxglove, Digitalis species Garlic, Allium sativum Climbing Vines: Golden ragwort, Senecio aureus Grape, Vitis vinifera Goldenrod, Solidago species Hops, Humulus lupulus Horehound, Marrubium vulgare Passion flower, Maypop, Passiflora incarnata Hyssop, Hyssopus officinalis Wild yam, Dioscorea villosa Joe Pye weed, Eupatorium purpureum Ladybells, Adenophora species Herbaceous Plants: Lady’s mantle, Alchemilla vulgaris Alfalfa, Medicago sativa Lavender, Lavendula angustifolia Aloe vera, Aloe barbadensis Lemon balm, Melissa officinalis American skullcap, Scutellaria laterifolia Licorice, Glycyrrhiza -

Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals As Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity
pathogens Article Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity Chrysa Androutsopoulou 1, Spyridoula D. Christopoulou 2, Panagiotis Hahalis 3, Chrysoula Kotsalou 1, Fotini N. Lamari 2 and Apostolos Vantarakis 1,* 1 Department of Public Health, Faculty of Medicine, University of Patras, 26504 Patras, Greece; [email protected] (C.A.); [email protected] (C.K.) 2 Laboratory of Pharmacognosy & Chemistry of Natural Products, Department of Pharmacy, University of Patras, 26504 Patras, Greece; [email protected] (S.D.C.); [email protected] (F.N.L.) 3 Tentoura Castro-G.P. Hahalis Distillery, 26225 Patras, Greece; [email protected] * Correspondence: [email protected] Abstract: Essential oils (EOs) and extracts of rose geranium (Pelargonium graveolens) and petals of rose (Rosa damascena) have been fully characterized in terms of composition, safety, antimicrobial, and antiviral properties. They were analyzed against Escherichia coli, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, Aspergillus niger, and Adenovirus 35. Their toxicity and life span were also determined. EO of P. graveolens (5%) did not retain any antibacterial activity (whereas at Citation: Androutsopoulou, C.; 100% it was greatly effective against E. coli), had antifungal activity against A. niger, and significant Christopoulou, S.D.; Hahalis, P.; antiviral activity. Rose geranium extract (dilutions 25−90%) (v/v) had antifungal and antibacterial Kotsalou, C.; Lamari, F.N.; Vantarakis, activity, especially against E. coli, and dose-dependent antiviral activity. Rose petals EO (5%) retains A. Evaluation of Essential Oils and low inhibitory activity against S. aureus and S. Typhimurium growth (about 20−30%), antifungal Extracts of Rose Geranium and Rose activity, and antiviral activity for medium to low virus concentrations. -

Seed Forgiveness of Some Species of the Family Lamiaceae Introduced in Tashkent Botanical Garden
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 7, Issue 11, 2020 SEED FORGIVENESS OF SOME SPECIES OF THE FAMILY LAMIACEAE INTRODUCED IN TASHKENT BOTANICAL GARDEN F.M.Dusmuratova1, T.Rakhimova2, D.K.Fakhriddinova3, A.I.Uralov4 1Department of Pharmacognosy Tashkent Pharmaceutical Institute, Tashkent, Uzbekistan. 2Biological faculty of the National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan. 3Tashkent Botanical Garden named after Acad. F.N. Rusanova at the Institute of Botany of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan. 4Jizzakh branch of the National University of Uzbekistan named after Mirzo Ulugbek, Uzbekistan. E-mail: [email protected] E-mail: [email protected] Abstract: The effects of temperature on seed germination, seed germination energy, and seed yield at different times were studied in the laboratory under 5 species (Origanum vulgare L., Lophanthus anisatus Benth., Hyssopus officinalis L., Lavandula officinalis Chaix., Salvia officinalis L.) belonging to the Lamiaceae family. It was also found that the seeds were lower in field conditions than the germination of seeds sown in autumn and spring. Therefore, it is recommended to sow the seeds of the studied species in the spring in the Uzbekistan climatic conditions. Keywords: Origanum vulgare, Lophanthus anisatus, Hyssopus officinalis, Lavandula officinalis, Salvia officinalis, seed,seed germination, germination energy. INTRODUCTION Today, the world's demand for herbal medicines is growing, and medicinal plants are widely used in medicine. Biologically active substances and extracts from plants are very popular in developed countries such as Japan, France, Germany and Italy. In many developing Asian countries, herbal medicines are important. -

GARDEN of the SUN - HERBS Page 1
GARDEN OF THE SUN - HERBS page 1 Botanical Name Common Name Achillea millefolium COMMON YARROW Achillea millefolium 'Rosea' DWARF PINK YARROW Achillea tomentosa WOOLLY YARROW Allium ampeloprasum ELEPHANT GARLIC Allium schoenoprasum CHIVES Aloysia citrodora LEMON VERBENA Artemisia abrotanum SOUTHERNWOOD Artemisia dracunculus FRENCH TARRAGON Brassica oleracea var. longata WALKING STICK KALE Buddleja BUTTERFLY BUSH Buxus BOXWOOD Cerastium tomentosum SNOW-IN-SUMMER Chaenomeles japonica 'Contorta' JAPANESE QUINCE 'CONTORTA' Cymbopogon citratus LEMON GRASS Digitalis purpurea FOXGLOVE Erigeron FLEABANE Eriophyllum confertiflorum GOLDEN YARROW Eruca 'Roquetta rugola' ARUGULA 'ROQUETTE RUGOLA' Eryngium SEA HOLLY Foeniculum vulgare COMMON FENNEL Gaillardia BLANKET FLOWER Lavandula LAVENDER Melissa officinalis LEMON BALM Micromeria thymifolia EMPEROR'S MINT Monarda BEE BALM Monarda didyma SCARLET BEE BALM Nepeta x faassenii CATMINT Origanum MOUNDING MARJORAM Origanum dictamnus DITTANY OF CRETE or HOP MARJORAM Origanum laevigatum 'Hopley's' ORNAMENTAL OREGANO 'HOPLEY'S' Origanum vulgare 'Aureum Crispum' GOLDEN CURLY OREGANO 'AUREUM CRISPUM' Origanum vulgare 'Creaton' OREGANO 'CREATON' Origanum vulgare var. hirtum GREEK OREGANO GARDEN OF THE SUN - HERBS page 2 Botanical Name Common Name Pelargonium APRICOT-SCENTED GERANIUM Pelargonium GINGER-SCENTED GERANIUM Pelargonium 'Attar of Roses' SCENTED GERANIUM 'ATTAR OF ROSES' Pelargonium 'Rober's Lemon Rose' GERANIUM 'ROBER'S LEMON ROSE' Pelargonium x hortorum 'Platinum' PLATINUM ZONAL or HORSESHOE GERANIUM -

Great Herbs for Kids Handout
Great Herbs for Kids The following is meant to be a general guide of herbs that are recommended for growing with children or placing in a children’s garden. For more detailed information, consult a reliable book on herb gardening, your local extension service, or your local garden center. Lemon Balm – Melissa officinalis Lemon Balm is easy to grow and maintain. In fact, take care with this plant because it is a prolific grower and can easily get out of control in the garden. Therefore, it is recommended that this herb be planted in a container. If you intend to put Lemon Balm in the ground be sure to remove the flower heads before they set seed. Children will enjoy the lemony fragrance of this plant as well as the texture of its leaves. Lemon Balm is easy to start from seed, making it an ideal pick for seed starting projects with your children. Light : full sun or partial shade Zone: hardy to zone 5 Growth: perennial Use: culinary, crafts Culture: well-drained, medium Landscape use: borders, containers rich soil, keep moist Sensory benefits: smell, touch, taste Sage – Salvia sp. Children will take great delight in watching the butterflies, birds, and bees that are attracted to these plants. Salvias also have fragrant leaves and striking flowers. Light : full sun Use: culinary, crafts Growth: perennial Landscape use: borders Soil: well-drained Sensory benefits: smell, visual, hearing Zone: hardy to zone 5 Good picks: Salvia elegans (pineapple sage), S. officinalis (garden sage) Sunflower – Helianthus annuus Sunflowers are often considered to somewhere between an herb and a food plant. -

Aloysia Citriodora Aloysia Citrodora Is a Species of Flowering Plant in the Verbena Family Verbenaceae, Native to Western South America
Aloysia citriodora Aloysia citrodora is a species of flowering plant in the verbena family Verbenaceae, native to western South America. Common names include lemon verbena and lemon beebrush. It was brought to Europe by the Spanish and the Portuguese in the 17th century and cultivated for its oil. Description Lemon verbena is a perennial shrub or subshrub growing to 2–3 m high. The 8-cm-long, glossy, pointed leaves are slightly rough to the touch and emit a powerful scent reminiscent of lemon when bruised (hence the Latin specific epithet citrodora—lemon-scented). Sprays of tiny purple or white flowers appear in late spring or early summer. It is sensitive to cold, losing leaves at temperatures below 0 °C (32 °F), although the wood is hardy to −10 °C (14 °F). Due to its many culinary uses, it is widely listed and marketed as a plant for the herb garden. Uses Lemon verbena leaves are used to add a lemon flavor to fish and poultry dishes, vegetable marinades, salad dressings, jams, puddings, Greek yogurt and beverages. It also is used to make herbal teas, or added to standard tea in place of actual lemon (as is common with Moroccan tea). It can also be used to make a sorbet. Chemistry The major isolates in lemon verbena oil are citral (30–35%), nerol and geraniol. Extracts of lemon verbena also contain verbascoside. Synonyms Synonyms for lemon verbena are Verbena triphylla L'Hér., Verbena citriodora Cav., Lippia triphylla, Lippia citriodora, Aloysia citriodora (Cav.) Ort.hierba luisa, cedron Garden history The first European botanist who publicly noticed this plant was the French Philibert Commerson, who collected in Buenos Aires on his botanical circumnavigation with Bougainville, about 1767. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Candidistat
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Candidistat Plants with Activity Synergy Chemical Count Total PPM Abies alba 1 2394.0 Abies balsamea 1 3100.0 Abies spectabilis 2 Acacia farnesiana 1 Achillea millefolium 2 340.0 Acinos suaveolens 3 Acorus calamus 3 27820.0 Aeolanthus myriantha 1 Aesculus hippocastanum 2 Agastache foeniculum 1 Agastache nepetoides 1 Agastache rugosa 1 Agastache urticifolia 1 4928.0 Ageratum conyzoides 1 Allium sativum var. sativum 2 Aloysia citrodora 2 2100.0 Alpinia galanga 2 Alpinia officinarum 2 Amomum compactum 2 Anacardium occidentale 1 Ananas comosus 1 Anethum graveolens 4 Angelica archangelica 3 2640.0 Annona squamosa 1 Apium graveolens 3 1438152.0 Armoracia rusticana 1 Artemisia annua 4 Plants with Activity Synergy Chemical Count Total PPM Artemisia dracunculus 3 Artemisia salsoloides 1 Artemisia vulgaris 1 Asarum canadense 1 Asiasarum sieboldii 1 Asimina triloba 1 1.096 Avena sativa 1 Ballota nigra 1 Boswellia sacra 1 Brassica oleracea var. capitata l. 1 Bursera delpechiana 1 720.0 Calamintha nepeta 1 Callicarpa americana 2 Camellia sinensis 2 Cananga odorata 1 Canarium indicum 1 1000.0 Capsicum annuum 2 Capsicum frutescens 2 Carica papaya 1 Carthamus tinctorius 1 0.04 Carum carvi 3 156060.0 Centella asiatica 2 Chamaemelum nobile 1 Chenopodium ambrosioides 1 Chrysanthemum balsamita 1 Chrysanthemum parthenium 1 Chrysanthemum x morifolium 1 2 Plants with Activity Synergy Chemical Count Total PPM Cinnamomum aromaticum 3 Cinnamomum camphora 1 240.0 Cinnamomum verum 3 384.0 Cistus