Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals As Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deodorizing Ability of Houttuynia Cordata Thunb. (Dokudami) for Masking Garlic Odor
www.ccsenet.org/jas Journal of Agricultural Science Vol. 4, No. 4; 2012 Deodorizing Ability of Houttuynia cordata Thunb. (Dokudami) for Masking Garlic Odor Hiromi Ikeura (Corresponding author) School of Agriculture, Meiji University 1-1-1, Higashimita, Tama-ku, Kawasaki, Kanagawa, 214-8571, Japan Tel: 81-44-934-7167 E-mail: [email protected] Received: December 6, 2011 Accepted: December 19, 2011 Online Published: February 2, 2012 doi:10.5539/jas.v4n4p245 URL: http://dx.doi.org/10.5539/jas.v4n4p245 Abstract The deodorizing activity of dokudami (HouttuyniacordataThunb.) for masking the odor of fresh garlic was investigated, and the volatility of the deodorizing compounds was examined. Two grams of garlic was placed in a beaker and heated for 5 min at 40 °C. Next, aliquots of 0.01 and 0.05 g of powdered samples of seven plants (dokudami, thyme, rosemary, coriander, dandelion, spiny sow thistle and green tea) were mixed with 1 ml of volatile or non-volatile dokudami extract and added to the garlic sample. The garlic odor intensity was subsequently evaluated by sniffing by 10 trained individuals. The deodorizing activity test was carried out at 0, 3 and 6 h after adding the dokudami extract. The deodorizing effect of dokudami on masking garlic odor was the highest of any plant and became higher with increased dose. In addition, the deodorizing activity of dokudami increased with time and persisted. Moreover, whether the deodorizing compounds of dokudami were volatile or non-volatile was investigated. The deodorizing activity of a volatile solution of dokudami against garlic was extremely higher than that of dokudami dry powder. -

Juniperus Communis L.) Essential Oil
Antioxidants 2014, 3, 81-98; doi:10.3390/antiox3010081 OPEN ACCESS antioxidants ISSN 2076-3921 www.mdpi.com/journal/antioxidants Article Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism Martina Höferl 1,*, Ivanka Stoilova 2, Erich Schmidt 1, Jürgen Wanner 3, Leopold Jirovetz 1, Dora Trifonova 2, Lutsian Krastev 4 and Albert Krastanov 2 1 Department of Pharmaceutical Chemistry, Division of Clinical Pharmacy and Diagnostics, University of Vienna, Vienna 1090, Austria; E-Mails: [email protected] (E.S.); [email protected] (L.J.) 2 Department Biotechnology, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mails: [email protected] (I.S.); [email protected] (D.T.); [email protected] (A.K.) 3 Kurt Kitzing Co., Wallerstein 86757, Germany; E-Mail: [email protected] 4 University Laboratory for Food Analyses, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +43-1-4277-55555; Fax: +43-1-4277-855555. Received: 11 December 2013; in revised form: 26 January 2014 / Accepted: 28 January 2014 / Published: 24 February 2014 Abstract: The essential oil of juniper berries (Juniperus communis L., Cupressaceae) is traditionally used for medicinal and flavoring purposes. As elucidated by gas chromatography/flame ionization detector (GC/FID) and gas chromatography/mass spectrometry (GC/MS methods), the juniper berry oil from Bulgaria is largely comprised of monoterpene hydrocarbons such as α-pinene (51.4%), myrcene (8.3%), sabinene (5.8%), limonene (5.1%) and β-pinene (5.0%). -

The Antibacterial and Antioxidant Effects of Clove (Syzygium Aromaticum) and Lemon Verbena (Aloysia Citriodora) Essential Oils
Journal of Human, Environment, and Health Promotion. 2019; 5(2): 86-93 Journal of Human, Environment, and Health Promotion Journal homepage: www.zums.ac.ir/jhehp The Antibacterial and Antioxidant Effects of Clove (Syzygium aromaticum) and Lemon Verbena (Aloysia citriodora) Essential Oils a b * c d Mahzad Hosseini Abdollah Jamshidi Mojtaba Raeisi Mohammad Azizzadeh a Student of Food Hygiene, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. b Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. c Department of Nutrition, Faculty of Health, Golestan University of Medical sciences, Gorgan, Iran. d Department of Clinical Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. *Corresponding author: Abdollah Jamshidi Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. Postal code: 9187195786. E-mail address: [email protected] ARTICLE INFO ABSTARACT Article type: Background: The study aimed to investigate the chemical composition, antimicrobial Original article effects, and antioxidant properties of clove and lemon verbena essential oils (EOs). Article history: Methods: The chemical composition of the EOs was identified using gas Received 31 March 2019 chromatography/mass spectrometry (GC/MS). In addition, the antibacterial effects of EOs Revised 9 June 2019 against seven important foodborne bacteria were assessed using the disk-diffusion, agar Accepted 20 June 2019 well-diffusion, and broth microdilution assays. Evaluation of the antioxidant properties of the EOs was carried out using DPPH, β-carotene-linoleic acid bleaching, and reducing DOI: 10.29252/jhehp.5.2.7 power assay. Keywords: Results: All the tested bacteria demonstrated susceptibility to EOs, with the highest Essential oil susceptibility observed in Bacillus cereus to the clove EO in the agar disk-diffusion test. -

An Overview on Phytopharmacology of Pelargonium Graveolens L
Indian Journal of Traditional Knowledge Vol. 14(4), October 2015, pp. 558-563 An overview on phytopharmacology of Pelargonium graveolens L. Jinous Asgarpanah1,2* & Fereshteh Ramezanloo2 1Young Researchers and Elite Club, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran 2Department of Pharmacognosy, Pharmaceutical Sciences Branch, Islamic Azad University (IAU), Tehran, Iran E-mail: [email protected] [email protected] Received 30 October 2014, revised 12 August 2015 Since ancient, Pelargonium graveolens L. is well organized for its therapeutic values. Only recently, its new medicinal aspects have been award by scientists. Regarding new multi-functional properties of P. graveolens and valuable ongoing reports we were prompted to update phytochemistry and pharmacology of it. Data were collected using of journals, articles, scientific books and websites such as Scopus and PubMed. P. graveolens extracts and essential oil are important in drug development with many pharmacologic properties in China and Middle East especially in Egypt and Morocco. P. graveolens has been used in traditional medicine for the relief of hemorrhoids, dysentery, inflammation and cancer, as well as in the perfumery, cosmetic and aromatherapy industries all over the world. P. graveolens has recently been shown to have antioxidant, antibacterial, antifungal activities and acaricidal effects. The valuable therapeutic aspects of P. graveolens are mostly correlated to the existence of volatile constituents, terpenoids and flavonoids. Due to being widespread and the easy collection of this plant and also remarkable biological activities and containing a high amount of essential oil, this plant has become a medicinal plant in pharmacy especially in aromatherapy. This overview presents comprehensive analyzed information on the phytochemical and clinical properties of P. -

Plants-Derived Biomolecules As Potent Antiviral Phytomedicines: New Insights on Ethnobotanical Evidences Against Coronaviruses
plants Review Plants-Derived Biomolecules as Potent Antiviral Phytomedicines: New Insights on Ethnobotanical Evidences against Coronaviruses Arif Jamal Siddiqui 1,* , Corina Danciu 2,*, Syed Amir Ashraf 3 , Afrasim Moin 4 , Ritu Singh 5 , Mousa Alreshidi 1, Mitesh Patel 6 , Sadaf Jahan 7 , Sanjeev Kumar 8, Mulfi I. M. Alkhinjar 9, Riadh Badraoui 1,10,11 , Mejdi Snoussi 1,12 and Mohd Adnan 1 1 Department of Biology, College of Science, University of Hail, Hail PO Box 2440, Saudi Arabia; [email protected] (M.A.); [email protected] (R.B.); [email protected] (M.S.); [email protected] (M.A.) 2 Department of Pharmacognosy, Faculty of Pharmacy, “Victor Babes” University of Medicine and Pharmacy, 2 Eftimie Murgu Square, 300041 Timisoara, Romania 3 Department of Clinical Nutrition, College of Applied Medical Sciences, University of Hail, Hail PO Box 2440, Saudi Arabia; [email protected] 4 Department of Pharmaceutics, College of Pharmacy, University of Hail, Hail PO Box 2440, Saudi Arabia; [email protected] 5 Department of Environmental Sciences, School of Earth Sciences, Central University of Rajasthan, Ajmer, Rajasthan 305817, India; [email protected] 6 Bapalal Vaidya Botanical Research Centre, Department of Biosciences, Veer Narmad South Gujarat University, Surat, Gujarat 395007, India; [email protected] 7 Department of Medical Laboratory, College of Applied Medical Sciences, Majmaah University, Al Majma’ah 15341, Saudi Arabia; [email protected] 8 Department of Environmental Sciences, Central University of Jharkhand, -

Influence of Drying Temperatures on Essential Oil Content in Savory Satureja Montana L
Influence of drying temperatures on essential oil content in savory Satureja montana L. (Lamiaceae ) Slavica Dudaš Polytechnic of Rijeka, Agricultural Department, Poreč, Croatia, [email protected] Petar Šegon Polytechnic of Rijeka, Agricultural Department, Poreč, Croatia Renata Erhatić College of Agriculture at Križevci, Croatia, [email protected] Vesna Kovačević Polytechnic of Rijeka, Agricultural Department, Poreč, Croatia, [email protected] Abstract Aromatic and medicinal plants like savory which produce essential oil and other active components are used in medicine and food processing industry as flavor, spice etc. On the market savory is present as dried herb, leaf drug, essential oil, extracts etc. Essential oil glands and trichomes of savory are located on the surface of stems, leaves and calyces, accordingly drying and processing of savory have huge influence on essential oil content in savory drugs. A research on wild growing savory from the Istrian peninsula was carried out at Polytechnic of Rijeka, Agricultural Department in Poreč. Drying kinetics and influence of drying temperatures (35, 40, 45, 50, 55 and 60°C) were investigated on selected parameters of savory collected in winter 2012, in the area of Rovinjsko selo in Istria, Croatia. The percentage of dried leaves in fresh herb, essential oil content and proportion of dried leaves in fresh and dried herb were defined. The average of initial water content was 80,06 %, essential oil content 0,776 ml100g -1 and the proportion of dried leaves was 50,56 % in the dried herb. Higher drying temperatures expectedly reduced the essential oil content. The reduction of essential oil content 425 at 45°C was 14,8 %, while further temperature increase to 50°C resulted with a 59,4 % lower content of essential oil in the drug. -

Salvia Officinalis L.), Petras R
286 Eur. J. Lipid Sci. Technol. 104 (2002) 286–292 Donata Bandoniene· a, Antioxidative activity of sage (Salvia officinalis L.), Petras R. Venskutonisa, Dainora Gruzdiene· a, savory (Satureja hortensis L.) and borage Michael Murkovicb (Borago officinalis L.) extracts in rapeseed oil The antioxidant activity (AA) of acetone oleoresins (AcO) and deodorised acetone a Department of Food extracts (DAE) of sage (Salvia officinalis L.), savory (Satureja hortensis L.) and borage Technology, Kaunas (Borago officinalis L.) were tested in refined, bleached and deodorised rapeseed oil University of Technology, applying the Schaal Oven Test and weight gain methods at 80 °C and the Rancimat Kaunas, Lithuania method at 120 °C. The additives (0.1 wt-%) of plant extracts stabilised rapeseed oil b Department of Food efficiently against its autoxidation; their effect was higher than that of the synthetic Chemistry and Technology, antioxidant butylated hydroxytoluene (0.02%). AcO and DAE obtained from the same Graz University of Technology, Graz, Austria herbal material extracted a different AA. The activity of sage and borage DAE was lower than that of AcO obtained from the same herb, whereas the AA of savory DAE was higher than that of savory AcO. The effect of the extracts on the oil oxidation rate measured by the Rancimat method was less significant. In that case higher concen- trations (0.5 wt-%) of sage and savory AcO were needed to achieve a more distinct oil stabilisation. Keywords: Antioxidant activity, sage, savory, borage, acetone oleoresin, deodorised acetone extract, rapeseed oil. 1 Introduction foods is a promising alternative to synthetic antioxidants [8]. Natural products isolated from spices and herbs can Lipid oxidation is a major cause for the deterioration of fat- act as antioxidants either solely or synergistically in mix- containing food. -

Research Paper the Effect of Salinity Stress and Temperature Levels
Academia Journal of Medicinal Plants 9(6): 070-077, June 2021 DOI: 10.15413/ajmp.2021.0107 ISSN: 2315-7720 ©2021 Academia Publishing Research Paper The effect of salinity stress and temperature levels on germination characteristics of four medicinal plants seed Accepted 9th March, 2021 ABSTRACT Cultivation of medicinal plants has been economically beneficial for pharmacy and medicine. These plants are stores of active and valuable secondary metabolites that can be converted into various drugs, some of which are life-saving. However, it is difficult to start a large-scale and commercial cultivation of these plants because most of the arable land is used to produce strategically essential crops. Other uncultivable lands are often affected by various abiotic stresses, one of the most important of which is salinity. Germination of plants is one of the important stages during their growth period that is often affected by environmental stresses, especially salinity. In this study, the seeds of medicinal plants such as Lavender (Lavandula angustifolia), hyssop (Hyssopus officinalis), black cumin (Nigella sativa L.) and Scrophularia (Scrophularia striata) were subjected to salinity stress at 20, 25 and 30°C in order to determine the germination characteristics of their seeds. The results of this experiment generally showed that with increasing salinity stress at different temperatures, all germination characteristics including germination percentage, germination rate, shoot length, root length, shoot dry weight, root dry weight and seed vigor index decreased. It seemed that in order to Yousef Hakimi grow plants commercially, low salinity soil and water are needed to get the best yield. Department of Horticulture, Faculty of Agriculture and Natural Resources, University Key words: Medicinal plants, salt stress, seed treatment, germination, lavender of Tehran, Karaj, Iran. -
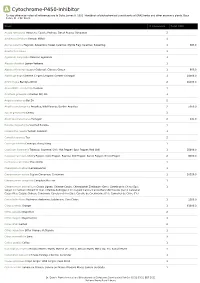
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Influence of Tea Tree Essential Oil and Poly(Ethylene Glycol)
materials Article Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films Iwona Tarach 1, Ewa Olewnik-Kruszkowska 1,* , Agnieszka Richert 2 , Magdalena Gierszewska 1 and Anna Rudawska 3 1 Chair of Physical Chemistry and Physicochemistry of Polymers, Faculty of Chemistry, Nicolaus Copernicus University in Toru´n,Gagarina 7 Street, 87-100 Toru´n,Poland; [email protected] (I.T.); [email protected] (M.G.) 2 Chair of Genetics, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University in Toru´n, Lwowska 1 Street, 87-100 Toru´n,Poland; [email protected] 3 Department of Production Engineering, Faculty of Mechanical Engineering, Lublin University of Technology, 20-618 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-611-2210 Received: 5 October 2020; Accepted: 1 November 2020; Published: 4 November 2020 Abstract: The aim of the study was to establish the influence of poly(ethylene glycol) (PEG) on the properties of potential biodegradable packaging materials with antibacterial properties, based on polylactide (PLA) and tea tree essential oil (TTO). The obtained polymeric films consisted of PLA, a natural biocide, and tea tree essential oil (5–20 wt. %) was prepared with or without an addition of 5 wt. % PEG. The PLA-based materials have been tested, taking into account their morphology, and their thermal, mechanical and antibacterial properties against Staphylococcus aureus and Escherichia coli. It was established that the introduction of a plasticizer into the PLA–TTO systems leads to an increase in tensile strength, resistance to deformation, as well an increased thermal stability, in comparison to films modified using only TTO. -

Melissa Officinalis L., a Valuable Medicine Plant: a Review
Journal of Medicinal Plants Research Vol. 4(25), pp. 2753-2759, 29 December Special Review, 2010 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2010 Academic Journals Review Melissa officinalis L., a valuable medicine plant: A review Moradkhani H.1, Sargsyan E.1, Bibak H.2, Naseri B.3, Sadat-Hosseini M.2, Fayazi-Barjin A.4 and Meftahizade H.5* 1Institute of Hydroponic Problems, National Academic of Sciences, Yerevan, Republic of Armenia. 2Department of plant production, faculty of Agriculture, university of Jiroft, Kerman, Iran. 3Faculty of Islamic Azad University, Ilam, Iran. 4Department of Plant Protection, University of Tehran, Iran. 5Researcher of ACECR Medicinal Plants Center, Ilam, Iran. Accepted 6 December, 2010 Melissa officinalis L., a valuable medicinal plant in herbal medicine is native to the eastern Mediterranean Region and western Asia. The constituent of the essential oil of the plant in various climates is different, but citral (geranial and neral), citronellal, geraniol are main components. Many parameters influencing essential oil composition and yield, such as light intensity, nutrient, temperature, cultural practice genotype, plant part age, harvesting time. Lemon balm has been traditionally used for different medical purposes as tonic, antispasmodic, carminative, diaphoretic, surgical dressing for wounds, sedative-hypnotic strengthening the memory, and relief of stress induced headache, but in modern pharmacology is value in the management of mild to moderate Alzheimer’s, against migraine and rheumatism, antitumel and antioxidant activities. Key words: Melissa officinalis, essential oil, pharmacology and antioxidant. INTRODUCTION Lemon balm, member of the family Lamiaceae (formerly years may no longer germinate (Zargari, 1991). Labiatae) is a perennial bushy plant and is upright, Lemon balm has a hairy root system with many lateral reaching a height of about 1 m. -

District Doings Gretchen Humphrey, PNW District Director
‘Catherine Graham’ Hybrid Tea Photo by Rich Baer In This Issue District Doings Gretchen Humphrey, PNW District Director You can email me at: [email protected] or call me at 503-539-6853 Message From the Director————— 1-2 District Horticulture Judging News—— 2-3 District CR Report—--———––——–- 3-4 Happy New Year to Everyone! District Show (Tri-City Rose Society)— 4 As we roll into a new year and a new growing season, I am excited to see what is Rose Science: Stomata: in store for us in the great Pacific Northwest. Windows to the Outside World-——– 5-6 Prizes and Awards ———————–- 7 Since our last newsletter, my husband and I traveled to the ARS National Con- Roses In Review————————— 8-9 vention in Tyler, Texas. This whirlwind weekend began with the Board Meeting on Rose Arrangement Workshop———– 10 Thursday, taking care of important ARS business. Following that was the Rose Show, Rose Arrangement School————— 11 held at the Rose Center in Tyler. This time, we didn’t bring any roses, since it was the Coming Events/Rose Show Dates—— 11 middle of October. Although that month was particularly dry, the timing of our blooms was off, and we didn’t have any worthy specimens. Old Garden Roses: The National Rose Show was rather small, although there were some beautiful What Are They?—–—————– 12-16 blooms, and some varieties we hadn’t seen before. After judging, we volunteered to guide Hybrid Gallicas——— 12-13 Damasks—————– 13-14 the busloads of visitors around the show. It turned out there weren’t that many on Friday, Albas——————— 14 but we did manage to greet a few nice folks.