Effects on Olive and Olive Oil Storage in the Tocopherols Content of Portuguese Virgin Olive Oils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Antibacterial and Antioxidant Effects of Clove (Syzygium Aromaticum) and Lemon Verbena (Aloysia Citriodora) Essential Oils
Journal of Human, Environment, and Health Promotion. 2019; 5(2): 86-93 Journal of Human, Environment, and Health Promotion Journal homepage: www.zums.ac.ir/jhehp The Antibacterial and Antioxidant Effects of Clove (Syzygium aromaticum) and Lemon Verbena (Aloysia citriodora) Essential Oils a b * c d Mahzad Hosseini Abdollah Jamshidi Mojtaba Raeisi Mohammad Azizzadeh a Student of Food Hygiene, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. b Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. c Department of Nutrition, Faculty of Health, Golestan University of Medical sciences, Gorgan, Iran. d Department of Clinical Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. *Corresponding author: Abdollah Jamshidi Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. Postal code: 9187195786. E-mail address: [email protected] ARTICLE INFO ABSTARACT Article type: Background: The study aimed to investigate the chemical composition, antimicrobial Original article effects, and antioxidant properties of clove and lemon verbena essential oils (EOs). Article history: Methods: The chemical composition of the EOs was identified using gas Received 31 March 2019 chromatography/mass spectrometry (GC/MS). In addition, the antibacterial effects of EOs Revised 9 June 2019 against seven important foodborne bacteria were assessed using the disk-diffusion, agar Accepted 20 June 2019 well-diffusion, and broth microdilution assays. Evaluation of the antioxidant properties of the EOs was carried out using DPPH, β-carotene-linoleic acid bleaching, and reducing DOI: 10.29252/jhehp.5.2.7 power assay. Keywords: Results: All the tested bacteria demonstrated susceptibility to EOs, with the highest Essential oil susceptibility observed in Bacillus cereus to the clove EO in the agar disk-diffusion test. -
Show Activity
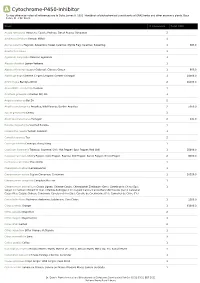
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Industrial Crops and Products 77 (2015) 972–982
Industrial Crops and Products 77 (2015) 972–982 Contents lists available at ScienceDirect Industrial Crops and Products journal homepage: www.elsevier.com/locate/indcrop Extending the use of irradiation to preserve chemical and bioactive properties of medicinal and aromatic plants: A case study with four species submitted to electron beam a a b a Eliana Pereira , Amilcar L. Antonio , Andrzej Rafalski , João C.M. Barreira , a a,∗ Lillian Barros , Isabel C.F.R. Ferreira a Centro de Investigac¸ ão de Montanha (CIMO), ESA, Instituto Politécnico de Braganc¸ a, Campus de Santa Apolónia, 1172, 5301-855 Braganc¸ a, Portugal b Centre for Radiation Research and Technology, Institute of Nuclear Chemistry and Technology, Dorodna Str. 16, 03-195 Warsaw, Poland a r t i c l e i n f o a b s t r a c t Article history: The effects of gamma irradiation on Aloysia citrodora, Melissa officinalis, Melittis melissophyllum and Mentha Received 28 June 2015 piperita were previously evaluated. Herein, the same species were treated with electron-beam irradia- Received in revised form tion (EB) and the same parameters were evaluated. Instead of presenting absolute values for each studied 24 September 2015 parameter, data were evaluated as percentage of induced variation. Besides the newly obtained results, Accepted 29 September 2015 data from a previous work was recalled and normalized in the same manner. Several examples of per- centage variations specific to a plant species or irradiation condition were found. Nevertheless, it was not Keywords: possible to identify unequivocal trends. Even so, when evaluated in an integrative way, the parameters Irradiation with highest discriminating ability among irradiation conditions or plant species were fatty acids and Aromatic plants bioactive indicators. -

Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals As Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity
pathogens Article Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity Chrysa Androutsopoulou 1, Spyridoula D. Christopoulou 2, Panagiotis Hahalis 3, Chrysoula Kotsalou 1, Fotini N. Lamari 2 and Apostolos Vantarakis 1,* 1 Department of Public Health, Faculty of Medicine, University of Patras, 26504 Patras, Greece; [email protected] (C.A.); [email protected] (C.K.) 2 Laboratory of Pharmacognosy & Chemistry of Natural Products, Department of Pharmacy, University of Patras, 26504 Patras, Greece; [email protected] (S.D.C.); [email protected] (F.N.L.) 3 Tentoura Castro-G.P. Hahalis Distillery, 26225 Patras, Greece; [email protected] * Correspondence: [email protected] Abstract: Essential oils (EOs) and extracts of rose geranium (Pelargonium graveolens) and petals of rose (Rosa damascena) have been fully characterized in terms of composition, safety, antimicrobial, and antiviral properties. They were analyzed against Escherichia coli, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, Aspergillus niger, and Adenovirus 35. Their toxicity and life span were also determined. EO of P. graveolens (5%) did not retain any antibacterial activity (whereas at Citation: Androutsopoulou, C.; 100% it was greatly effective against E. coli), had antifungal activity against A. niger, and significant Christopoulou, S.D.; Hahalis, P.; antiviral activity. Rose geranium extract (dilutions 25−90%) (v/v) had antifungal and antibacterial Kotsalou, C.; Lamari, F.N.; Vantarakis, activity, especially against E. coli, and dose-dependent antiviral activity. Rose petals EO (5%) retains A. Evaluation of Essential Oils and low inhibitory activity against S. aureus and S. Typhimurium growth (about 20−30%), antifungal Extracts of Rose Geranium and Rose activity, and antiviral activity for medium to low virus concentrations. -

GARDEN of the SUN - HERBS Page 1
GARDEN OF THE SUN - HERBS page 1 Botanical Name Common Name Achillea millefolium COMMON YARROW Achillea millefolium 'Rosea' DWARF PINK YARROW Achillea tomentosa WOOLLY YARROW Allium ampeloprasum ELEPHANT GARLIC Allium schoenoprasum CHIVES Aloysia citrodora LEMON VERBENA Artemisia abrotanum SOUTHERNWOOD Artemisia dracunculus FRENCH TARRAGON Brassica oleracea var. longata WALKING STICK KALE Buddleja BUTTERFLY BUSH Buxus BOXWOOD Cerastium tomentosum SNOW-IN-SUMMER Chaenomeles japonica 'Contorta' JAPANESE QUINCE 'CONTORTA' Cymbopogon citratus LEMON GRASS Digitalis purpurea FOXGLOVE Erigeron FLEABANE Eriophyllum confertiflorum GOLDEN YARROW Eruca 'Roquetta rugola' ARUGULA 'ROQUETTE RUGOLA' Eryngium SEA HOLLY Foeniculum vulgare COMMON FENNEL Gaillardia BLANKET FLOWER Lavandula LAVENDER Melissa officinalis LEMON BALM Micromeria thymifolia EMPEROR'S MINT Monarda BEE BALM Monarda didyma SCARLET BEE BALM Nepeta x faassenii CATMINT Origanum MOUNDING MARJORAM Origanum dictamnus DITTANY OF CRETE or HOP MARJORAM Origanum laevigatum 'Hopley's' ORNAMENTAL OREGANO 'HOPLEY'S' Origanum vulgare 'Aureum Crispum' GOLDEN CURLY OREGANO 'AUREUM CRISPUM' Origanum vulgare 'Creaton' OREGANO 'CREATON' Origanum vulgare var. hirtum GREEK OREGANO GARDEN OF THE SUN - HERBS page 2 Botanical Name Common Name Pelargonium APRICOT-SCENTED GERANIUM Pelargonium GINGER-SCENTED GERANIUM Pelargonium 'Attar of Roses' SCENTED GERANIUM 'ATTAR OF ROSES' Pelargonium 'Rober's Lemon Rose' GERANIUM 'ROBER'S LEMON ROSE' Pelargonium x hortorum 'Platinum' PLATINUM ZONAL or HORSESHOE GERANIUM -

Great Herbs for Kids Handout
Great Herbs for Kids The following is meant to be a general guide of herbs that are recommended for growing with children or placing in a children’s garden. For more detailed information, consult a reliable book on herb gardening, your local extension service, or your local garden center. Lemon Balm – Melissa officinalis Lemon Balm is easy to grow and maintain. In fact, take care with this plant because it is a prolific grower and can easily get out of control in the garden. Therefore, it is recommended that this herb be planted in a container. If you intend to put Lemon Balm in the ground be sure to remove the flower heads before they set seed. Children will enjoy the lemony fragrance of this plant as well as the texture of its leaves. Lemon Balm is easy to start from seed, making it an ideal pick for seed starting projects with your children. Light : full sun or partial shade Zone: hardy to zone 5 Growth: perennial Use: culinary, crafts Culture: well-drained, medium Landscape use: borders, containers rich soil, keep moist Sensory benefits: smell, touch, taste Sage – Salvia sp. Children will take great delight in watching the butterflies, birds, and bees that are attracted to these plants. Salvias also have fragrant leaves and striking flowers. Light : full sun Use: culinary, crafts Growth: perennial Landscape use: borders Soil: well-drained Sensory benefits: smell, visual, hearing Zone: hardy to zone 5 Good picks: Salvia elegans (pineapple sage), S. officinalis (garden sage) Sunflower – Helianthus annuus Sunflowers are often considered to somewhere between an herb and a food plant. -

Aloysia Citriodora Aloysia Citrodora Is a Species of Flowering Plant in the Verbena Family Verbenaceae, Native to Western South America
Aloysia citriodora Aloysia citrodora is a species of flowering plant in the verbena family Verbenaceae, native to western South America. Common names include lemon verbena and lemon beebrush. It was brought to Europe by the Spanish and the Portuguese in the 17th century and cultivated for its oil. Description Lemon verbena is a perennial shrub or subshrub growing to 2–3 m high. The 8-cm-long, glossy, pointed leaves are slightly rough to the touch and emit a powerful scent reminiscent of lemon when bruised (hence the Latin specific epithet citrodora—lemon-scented). Sprays of tiny purple or white flowers appear in late spring or early summer. It is sensitive to cold, losing leaves at temperatures below 0 °C (32 °F), although the wood is hardy to −10 °C (14 °F). Due to its many culinary uses, it is widely listed and marketed as a plant for the herb garden. Uses Lemon verbena leaves are used to add a lemon flavor to fish and poultry dishes, vegetable marinades, salad dressings, jams, puddings, Greek yogurt and beverages. It also is used to make herbal teas, or added to standard tea in place of actual lemon (as is common with Moroccan tea). It can also be used to make a sorbet. Chemistry The major isolates in lemon verbena oil are citral (30–35%), nerol and geraniol. Extracts of lemon verbena also contain verbascoside. Synonyms Synonyms for lemon verbena are Verbena triphylla L'Hér., Verbena citriodora Cav., Lippia triphylla, Lippia citriodora, Aloysia citriodora (Cav.) Ort.hierba luisa, cedron Garden history The first European botanist who publicly noticed this plant was the French Philibert Commerson, who collected in Buenos Aires on his botanical circumnavigation with Bougainville, about 1767. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Candidistat
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Candidistat Plants with Activity Synergy Chemical Count Total PPM Abies alba 1 2394.0 Abies balsamea 1 3100.0 Abies spectabilis 2 Acacia farnesiana 1 Achillea millefolium 2 340.0 Acinos suaveolens 3 Acorus calamus 3 27820.0 Aeolanthus myriantha 1 Aesculus hippocastanum 2 Agastache foeniculum 1 Agastache nepetoides 1 Agastache rugosa 1 Agastache urticifolia 1 4928.0 Ageratum conyzoides 1 Allium sativum var. sativum 2 Aloysia citrodora 2 2100.0 Alpinia galanga 2 Alpinia officinarum 2 Amomum compactum 2 Anacardium occidentale 1 Ananas comosus 1 Anethum graveolens 4 Angelica archangelica 3 2640.0 Annona squamosa 1 Apium graveolens 3 1438152.0 Armoracia rusticana 1 Artemisia annua 4 Plants with Activity Synergy Chemical Count Total PPM Artemisia dracunculus 3 Artemisia salsoloides 1 Artemisia vulgaris 1 Asarum canadense 1 Asiasarum sieboldii 1 Asimina triloba 1 1.096 Avena sativa 1 Ballota nigra 1 Boswellia sacra 1 Brassica oleracea var. capitata l. 1 Bursera delpechiana 1 720.0 Calamintha nepeta 1 Callicarpa americana 2 Camellia sinensis 2 Cananga odorata 1 Canarium indicum 1 1000.0 Capsicum annuum 2 Capsicum frutescens 2 Carica papaya 1 Carthamus tinctorius 1 0.04 Carum carvi 3 156060.0 Centella asiatica 2 Chamaemelum nobile 1 Chenopodium ambrosioides 1 Chrysanthemum balsamita 1 Chrysanthemum parthenium 1 Chrysanthemum x morifolium 1 2 Plants with Activity Synergy Chemical Count Total PPM Cinnamomum aromaticum 3 Cinnamomum camphora 1 240.0 Cinnamomum verum 3 384.0 Cistus -

Garden of the Sun Herbs April, 2017
Garden of the Sun Herbs April, 2017 # BOTANICAL NAME COMMON NAME Achillea millefolium COMMON YARROW Achillea millefolium 'Rosea' DWARF PINK YARROW Achillea tomentosa WOOLLY YARROW Aloysia citrodora LEMON VERBENA Artemisia abrotanum SOUTHERNWOOD Arugula (Eruca) 'Roquetta rugola' ARUGULA 'ROQUETTE RUGOLA' Brassica oleracea var. longata WALKING STICK KALE Buddleja BUTTERFLY BUSH Buxus BOXWOOD Cerastium tomentosum SNOW-IN-SUMMER Chaenomeles japonica 'Contorta' CONTORTED JAPANESE QUINCE Chives (Allium schoenoprasum) CHIVES Cymbopogon citratus LEMON GRASS Digitalis purpurea FOXGLOVE Erigeron FLEABANE Eriophyllum confertiflorum GOLDEN YARROW Eryngium SEA HOLLY Fennel (Foeniculum vulgare) COMMON FENNEL 2 Gaillardia BLANKET FLOWER Garlic (Allium ampeloprasum) ELEPHANT GARLIC Lavandula LAVENDER Melissa officinalis LEMON BALM 2 Micromeria thymifolia EMPEROR'S MINT Monarda BEE BALM Monarda didyma SCARLET BEE BALM Nepeta x faassenii CATMINT Oregano (Origanum dictamnus) DITTANY OF CRETE or HOP MARJORAM Oregano (Origanum vulgare var. hirtum) GREEK OREGANO Oregano (Origanum vulgare) 'Aureum Crispum' GOLDEN CURLY OREGANO Oregano (Origanum vulgare) 'Creaton' OREGANO 'CREATON' Origanum MOUNDING MARJORAM Origanum laevigatum 'Hopley's' ORNAMENTAL OREGANO 'HOPLEY'S' Parsley, Flat Leaf (Petroselinum crispum) FLAT LEAF or ITALIAN PARSLEY Pelargonium APRICOT-SCENTED GERANIUM Pelargonium GINGER-SCENTED GERANIUM 2 Pelargonium 'Attar of Roses' SCENTED GERANIUM 'ATTAR OF ROSES' Pelargonium 'Rober's Lemon Rose' GERANIUM 'ROBER'S LEMON ROSE' Pelargonium x hortorum -

Annual Plant Sale
Annual Plant Sale May 17, 18 & 19, 2019 Vegetables Cabbage Famosa, Early Bird, Amarant Chard Rainbow Cucumber Marketmore 76, Boston Pickler, *Shintokiwa Eggplant *Ping Tung Long Ground Cherry Kale Lacinato, Red Russian, Ruffles Lettuce Year-Round Bronze Oak, Speckled Amish Butterhead, *Canasta, Magenta, Victoria, Jericho, Craco Peppers (Hot) Bolivian Rainbow, Aji Crystal, Ring of Fire Cayenne, Jalapeño Peppers (Sweet) Lipstick, Jimmy Nardello, King of the North, Sullivan’s Italian Frying, Yankee Bell, Orange Bell, Golden California Purple Tomatillo Heirloom Squash (Summer) Dark Green Zucchini, Success Yellow Straightneck Squash (Winter) *Butternut, Baby Pam Pumpkin Zucchini (See Summer Squash) Tomatoes Cherry Sungold, *Snow White, Supersweet 100, *Blush, California Sunshine, Pink Bumblebee, Black Cherry Paste San Marzano, Amish Paste Slicing (Red) Celebrity, *Ruth’s Perfect, Brandywine (Heirloom), Defiant, Robbin’s Red, Cherokee Purple (heirloom) Slicing (Non-Red) *Persimmon, Green Zebra, Indigo Apple *Asterisk indicates plants grown from seeds saved at the Pfeiffer Center. Hours: May 17 &18: 9:00am-5:00pm; May 19: 9:00am-4:00pm At the Pfeiffer Center Garden, opposite 285 Hungry Hollow Rd., Chestnut Ridge, NY 10977 For information: [email protected] 845-352-5020 x124 www.pfeiffercenter.org Annual Plant Sale May 17, 18 & 19, 2019 Herbs, Perennial Anise Hyssop Agastache foeniculum Hyssop Hyssopus officinalis Lavender Munstead Lavendula augustifolia Lemon Balm Melissa officinalis Lemon Verbena Aloysia citrodora Lovage Levisticum officinale -

Bring a Wagon! NATIVE PLANTS See Natives List 01 NATIVE PLANTS See Natives List 02 NATIVE PLANTS See Natives List 03
NEW ORLEANS BOTANICAL GARDEN PELICAN GREENHOUSE SALE June 15, 2019 9 am – NOON Greenhouse is located just off Henry Thomas (Golf) Drive south of the I 610 underpass, Google Maps Address – 2 Celebration Drive Bring a wagon! NATIVE PLANTS See Natives list 01 NATIVE PLANTS See Natives list 02 NATIVE PLANTS See Natives list 03 Aloysia citrodora Lemon Verbena 04 Artemisia absinthium Wormwood 04 Cymbopogon sp Lemongrass 04 EGGPLANT Solanum melongena ‘Patio Baby’ Patio Baby Eggplant 04 Ficus carica ‘Celeste’ Celeste Fig 04 Monarda didyma ‘Jacob Cline’ Jacob Cline Beebalm 04 Moringa oleifera Drumstick Tree 04 Nashia inaguensis Bahama Berry 04 Nepeta cataria citriodora Lemon Catnip 04 Punica granatum ‘Wonderful’ Pomegranate 04 Rosmarinus officinalis ‘Huntington’s Carpet’ Rosemary 04 TOMATO Valley Girl 04 Agastache foeniculum Anise Hyssop 05 Aloysia virgata Sweet Verbena 05 Brilliantasia subulugarica Big Blue Salvia 05 Cuphea schumannii Orange Cuphea 05 Dicliptera squarrosa Hummingbird Plant 05 Echinacea ‘Tomato Soup’ Tomato Soup Coneflower 05 Hamelia patens Firebush 05 Hemerocallis Aztec Gold 05 Hemerocallis Little Business 05 Lagerstroemia ‘Cherry Mocha’ Cherry Mocha Crape Myrtle 05 Phlox ‘Robert Poore’ Robert Poore Phlox 05 Rudbeckia ‘ Early Bird Gold’ Early Bird Gold Coneflower 05 Stachytarpheta mutabilis Pink Porterweed 05 Tecoma Stans Yellow Bells 05 Verbena ‘Snow Flurries’ Snow Flurries Verbena 05 Salvia ‘El Cielo Blue’ Blue Sky Sage 06 Salvia ‘Phyllis’ Fancy’ Phyllis’ Fancy Sage 06 Salvia elegans Pineapple Sage 06 Salvia guaranitica ‘Blue -

Herbs and Flowers Available at the 2021 WCG Plant Sale Jump To: Herbs, Flowers, Ground Covers and Grasses
Herbs and Flowers Available at the 2021 WCG Plant Sale Jump to: Herbs, Flowers, Ground Covers and Grasses Herbs Genus and Variety Common Name Description Easy to grow and tolerant to drought. Catnip pleasantly stimulates your cat's pheromone receptors, resulting in temporary euphoria. Can also be used by humans to make a warm, minty tea that is used to induce sleep and said to Nepeta Cataria Catnip relieve cold symptoms. Can be a very invasive, to keep it from overrunning try growing it in a container. Roman Chamomile can be used to make a fragrant pathway or a nice, aromatic surprise tucked among other garden plants. If it pushes against other plants, it can get up to a foot high with bloom. It can also be mowed to the ground to Chamomile Roman Chamomile keep it flat. However, it is an important beneficial insect plant so leaving those flowers on may be a better choice! The flat, tender leaves have a flavor midway between garlic and onion. Delicious in salads, spreads and flavored vinegars. Perennial in Zones 3-10. Include garlic chives in perennial borders, herb gardens, vegetable and containers, indoors and Allium tuberosum Garlic Chives out. Chives are cool-season, cold-tolerant perennials that are planted in early spring. Be mindful when planting this herb, as it will take over your garden if the flowers are left to ripen (the flowers scatter the seeds). However, this plant is easy to dig Allium schoenoprasum Chives up and move if it overwhelms your garden. Annual herb used in cuisines around the world.