Exposure Assessment and the Risk Associated with Trihalomethane Compounds in Drinking Water, Cairo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2016|2017 2016|2017
Egyypt 2016|2017 Discovering Business in association with Copyright © Allurentis Limited 2016. All rights reserved. Allurentis is delighted to have been involved in association with UK Trade & Investment on this, the first edition of Egypt - Discovering Business and would like to thank all sponsoring organisations for their kind contributions. We are confident that it will raise awareness with all readers and prove to be an invaluable resource, especially for those wishing to become involved in the extraordinary business opportunities and growth prospects within Egypt. Electronic copies of this publication may be downloaded from Allurentis Limited's website at www.allurentis.com, provided that the use of any copy so downloaded, complies with the terms and conditions specified on the website. Except as expressly stated above, no part of this publication may be copied, reproduced, stored or transmitted in any form or by any means without the prior permission in writing from Allurentis Limited. To enquire about obtaining permission for uses other than those permitted above, please contact Allurentis by sending an email to [email protected] Photos courtesy of: www.istockphoto.com & www.123rf.com USINESS B Contents ISCOVERING Introduction Egypt turns to private sector to build new economy 5 D - 2016|2017 Messages GYPT E H.E. Ambassador Nasser Kamel: Egyptian Ambassador to the UK 8 HMA John Casson: British Ambassador to Egypt 9 UK Trade & Investment in Egypt 10 Egyptian Commercial Service in the UK 11 Business - Legal - Finance Egypt’s economic -

Mints – MISR NATIONAL TRANSPORT STUDY
No. TRANSPORT PLANNING AUTHORITY MINISTRY OF TRANSPORT THE ARAB REPUBLIC OF EGYPT MiNTS – MISR NATIONAL TRANSPORT STUDY THE COMPREHENSIVE STUDY ON THE MASTER PLAN FOR NATIONWIDE TRANSPORT SYSTEM IN THE ARAB REPUBLIC OF EGYPT FINAL REPORT TECHNICAL REPORT 11 TRANSPORT SURVEY FINDINGS March 2012 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS CO., LTD. ALMEC CORPORATION EID KATAHIRA & ENGINEERS INTERNATIONAL JR - 12 039 No. TRANSPORT PLANNING AUTHORITY MINISTRY OF TRANSPORT THE ARAB REPUBLIC OF EGYPT MiNTS – MISR NATIONAL TRANSPORT STUDY THE COMPREHENSIVE STUDY ON THE MASTER PLAN FOR NATIONWIDE TRANSPORT SYSTEM IN THE ARAB REPUBLIC OF EGYPT FINAL REPORT TECHNICAL REPORT 11 TRANSPORT SURVEY FINDINGS March 2012 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS CO., LTD. ALMEC CORPORATION EID KATAHIRA & ENGINEERS INTERNATIONAL JR - 12 039 USD1.00 = EGP5.96 USD1.00 = JPY77.91 (Exchange rate of January 2012) MiNTS: Misr National Transport Study Technical Report 11 TABLE OF CONTENTS Item Page CHAPTER 1: INTRODUCTION..........................................................................................................................1-1 1.1 BACKGROUND...................................................................................................................................1-1 1.2 THE MINTS FRAMEWORK ................................................................................................................1-1 1.2.1 Study Scope and Objectives .........................................................................................................1-1 -

Analysis of Land Use Land Cover Change in Greater Cairo Region: 1984-2016
Master Thesis Submitted within the UNIGIS MSc. programme at the Department of Geoinformatics - Z_GIS University of Salzburg, Austria Under the provisions of UNIGIS India framework Analysis of Land Use Land Cover Change in Greater Cairo Region: 1984-2016 by Ahmed Omar Shehata Omar GIS_104409 A thesis submitted in partial fulfillment of the requirements of the degree of Master of Science (Geographical Information Science & Systems) – MSc (GISc) Advisor (s): Dr. Shahnawaz Cairo-Egypt, 18-11-2017 Science Pledge By my signature below, I certify that my thesis is entirely the result of my own work. I have cited all sources I have used in my thesis and I have always indicated their origin. Cairo-Egypt, 18-11-2017 Ahmed Omar 1 Acknowledgements I would like to thank my thesis advisor Dr. Shahnawaz for his great academic and moral support especially in the most difficult times. As I cannot but appreciate the constructive suggestions, criticisms and encouragement. He consistently allowed this thesis to be my own work, also steered me in the right the direction whenever he thought I needed it. 2 Abstract The Greater Cairo Region (GCR) is one of the most intensively populated areas in the world, one of the fastest growing mega cities in the world. This place an ever increasing need for urban development to accommodate such population growth in both residential complexes and work facilities. Since 1980th, rapid population growth and urbanization have become issues in big cities in developing countries like Greater Cairo. As a consequence of explosive growth, the living conditions of Greater Cairo deteriorate (Cairo, Giza, and Qalyubia). -

GEORGIA – EGYPT Economic Development Connection
GEORGIA – EGYPT Economic Development Connection Government & Commerce partnership agreements with Egyptian schools such as Ain Shams University, Alexandria The Embassy of the Arab Republic of Egypt, University, Helwan University and the Egyptian located in Washington, D.C., has jurisdiction over University Sports Federation. These partnerships the states of Georgia, Delaware, Florida, focus on student and faculty exchanges as well Maryland, North Carolina, South Carolina, Virginia as joint research efforts especially for and West Virginia. Mr. Mohamed Tawfik has international grants. served as Egypt’s Ambassador to the United Georgia State University has a cooperation States since September 2012. agreement with Egypt’s Cairo University to help The Atlanta Civic Center, in partnership with build degree programs in business and nursing Emory University’s Michael C. Carlos Museum, and facilitate collaboration in curriculum hosted the King Tut exhibition in 2008. It innovation, teaching, research and service. attracted hundreds of visitors to Georgia and had The University System of Georgia offers four a very positive economic impact. The Michael C. study abroad programs to Egypt, including a Carlos Museum has a permanent collection of history program traveling to Cairo, Alexandria ancient Egyptian art. and Luxor and an Arabic language program in In 2009 the Egypt development group, Hands Cairo. Along the Nile, hosted a dialogue event on the role of the media in relations between the Nile Trade Relationship region and the United States. EXPORTS: In 2012, Georgia exports to Egypt The Atlanta chapter of the Egypt Cancer totaled $170 million. Egypt is currently the 40th Network hosted a gala in May of 2012 at the Fox largest export market for Georgia. -

Information for Asylum-Seekers and Refugees in Egypt
UNHCR The UN Refugee Agency Information For Asylum-Seekers and Refugees in Egypt United Nations High Commissioner for Refugees Regional Representation in Egypt Cairo, April 2013 CONTENTS Page INTRODUCTION 4 PART ONE: UNHCR MANDATE AND ITS ROLE IN THE ARAB REPUBLIC OF EGYPT 5 1.1 UNHCR Mandate 5 1.2 UNHCR Role in the Arab Republic of Egypt 7 PART TWO: RECEPTION AND GENERAL OFFICE PROCEDURES 11 2.1 Reception 11 2.2 General Office Procedures 13 2.3 Code of Conduct 18 PART THREE: REGISTRATION AND DOCUMENTATION FOR REFUGEES AND ASYLUM SEEKERS 22 3.1 Registration Process 22 3.2 Documentation-Process 29 PART FOUR: REFUGEE STATUS DETERMINATION PROCESS 42 4.1 Refugee Status Determination (RSD interview) 42 4.2 Legal Aid / Representation 45 4.3 Notification of RSD decisions 46 2 4.4 Appeal process 50 4.5 Cancellation and cessation of refugee status 54 4.6 Re-opening requests 56 4.7 Family unity 58 PART FIVE: LEGAL PROTECTION 64 PART SIX: ACCESS TO ASYLUM RIGHTS 66 6.1 Access to Health Care 66 6.2 Access to Education 73 6.3 Psycho-Social support at community level 79 6.4 Access to community based services 81 PART SEVEN: MEANS OF LIVELIHOOD 83 7.1 Means of live lihood 83 7.2 Vocational training 85 PART EIGHT: FINANCIAL ASSISTANCE 87 PART NINE: DURABLE SOLUTIONS 90 9.1 Voluntary Repatriation 90 9.1.1 Return to South Sudan 94 9.1.2 Return to the Sudan 97 9.1.3 Return to Iraq 98 9.2 Local Integration 101 9.3 Resettlement 102 PART TEN: UNHCR CAIRO COMPLAINTS PROCEDURES 109 PART ELEVEN: USEFUL CONTACTS 113 3 INTRODUCTION The purpose of this information booklet is to provide an overview of the mandate of the United Nations High Commissioner for Refugees (UNHCR) and the relevant criteria and procedures that are implemented by UNHCR in Egypt. -

World Bank Urban Transport Strategy Review Reportbird-Eng1.Doc Edition 3 – Nov
Public Disclosure Authorized Edition Date Purpose of edition / revision 1 July 2000 Creation of document – DRAFT – Version française 2 Sept. 2000 Final document– French version 3 Nov. 2000 Final document – English version EDITION : 3 Name Date Signature Public Disclosure Authorized Written by : Hubert METGE Verified by : Alice AVENEL Validated by Hubert METGE It is the responsibility of the recipient of this document to destroy the previous edition or its relevant copies WORLD BANK URBAN TRANSPORT Public Disclosure Authorized STRATEGY REVIEW THE CASE OF CAIRO EGYPT Public Disclosure Authorized Ref: 3018/SYS-PLT/CAI/709-00 World bank urban transport strategy review Reportbird-Eng1.doc Edition 3 – Nov. 2000 Page 1/82 The case of Cairo – Egypt WORLD BANK URBAN TRANSPORT STRATEGY REVIEW THE CASE OF CAIRO EGYPT EXECUTIVE SUMMARY Ref: 3018/SYS-PLT/CAI/709-00 World bank urban transport strategy review Reportbird-Eng1.doc Edition 3 – Nov. 2000 Page 2/82 The case of Cairo – Egypt EXECUTIVE SUMMARY TABLE OF CONTENTS 3 A) INTRODUCTION ....................................................................................................................................4 B) THE TRANSPORT POLICY SINCE 1970..................................................................................................4 C) CONSEQUENCES OF THE TRANSPORT POLICY ON MODE SPLIT.............................................................6 D) TRANSPORT USE AND USER CATEGORIES .............................................................................................7 E) TRANSPORT -

Analysis of the Surface Air Quality Measurements in the Greater Cairo (Egypt) Metropolitan
Vol-5, Issue-6 PP. 207-214 ISSN: 2394-5788 ANALYSIS OF THE SURFACE AIR QUALITY MEASUREMENTS IN THE GREATER CAIRO (EGYPT) METROPOLITAN Amira N. Mostafa & Ashraf S. Zakey Egyptian Meteorological Authority Cairo, Egypt [email protected] , [email protected] A. Soltan Monem M. Magdy Abdel Wahab Biophysics Department, Faculty of Science, Astronomy and Meteorology Department, Cairo University Cairo, Faculty of Science, Cairo University, Cairo, Egypt. Egypt. [email protected] [email protected] ABSTRACT Air pollution is considered one of the most severe issues facing developing countries. The Greater Cairo (GC) region has long been suffering from deteriorated air quality which is caused by high levels of anthropogenic activities, such as traffic, industries, and agricultural biomass burning events, as well as natural sources of particulate matter, such as dust and sand events. The present study aims at studying environmental pollution in GC area. The emission patterns of some major pollutants (sulfur dioxide, nitrogen dioxide, carbon monoxide, ozone, and particulate matter) measured by several pollution stations distributed around GC were analyzed, and areas of pollution hot spots were identified. The pollutants concentrations were found to exceed remarkably the Egyptian legal exposure limits during the period of measurement in some industrial areas. More actions need to be taken in order to lower the levels of air pollution and enhance the quality of life in the GC metropolitan. General Terms: Air Quality; Air Pollution Keywords: Air Quality; Air Pollution; Emission Pattern of Air Pollutants; Greater Cairo (Egypt) 1. INTRODUCTION Air pollution is the introduction of particulates or harmful substances which are not part of the natural composition of air into the Earth's atmosphere, causing disease, death, damage, or disruption of the natural ecosystem. -

Resistant Escherichia Coli: a Risk to Public Health and Food Safety
www.nature.com/scientificreports OPEN Poultry hatcheries as potential reservoirs for antimicrobial- resistant Escherichia coli: A risk to Received: 12 September 2017 Accepted: 21 March 2018 public health and food safety Published: xx xx xxxx Kamelia M. Osman1, Anthony D. Kappell2, Mohamed Elhadidy3,4, Fatma ElMougy5, Wafaa A. Abd El-Ghany6, Ahmed Orabi1, Aymen S. Mubarak7, Turki M. Dawoud7, Hassan A. Hemeg8, Ihab M. I. Moussa7, Ashgan M. Hessain9 & Hend M. Y. Yousef10 Hatcheries have the power to spread antimicrobial resistant (AMR) pathogens through the poultry value chain because of their central position in the poultry production chain. Currently, no information is available about the presence of AMR Escherichia coli strains and the antibiotic resistance genes (ARGs) they harbor within hatchezries. Therefore, this study aimed to investigate the possible involvement of hatcheries in harboring hemolytic AMR E. coli. Serotyping of the 65 isolated hemolytic E. coli revealed 15 serotypes with the ability to produce moderate bioflms, and shared susceptibility to cephradine and fosfomycin and resistance to spectinomycin. The most common β-lactam resistance gene was blaTEM, followed by blaOXA-1, blaMOX-like, blaCIT-like, blaSHV and blaFOX. Hierarchical clustering of E. coli isolates based on their phenotypic and genotypic profles revealed separation of the majority of isolates from hatchlings and the hatchery environments, suggesting that hatchling and environmental isolates may have diferent origins. The high frequency of β-lactam resistance genes in AMR E. coli from chick hatchlings indicates that hatcheries may be a reservoir of AMR E. coli and can be a major contributor to the increased environmental burden of ARGs posing an eminent threat to poultry and human health. -

Towards a Safer City – Sexual Harassment in Greater Cairo
Towards A Safer City Sexual Harassment in Greater Cairo: Effectiveness of Crowdsourced Data HarassMap conducted this research in collaboration with Youth and Development Consultancy Institute (Etijah). The study was supported by the International Development Research Center (IDRC) . The Additional information about the research can be obtained from the link below www.harassmap.org Copyrights © HarassMap 2014 Dep. No: 2014/13131 Printing house: Promotion Team Cover Design& Layout: Author and Study Principle Investigator Study Advisory Team Amel Fahmy Helen Rizzo Co-authors Maia Sieverding Angie Abdelmonem Fatan Abdel-Fatah Enas Hamdy Editorial Team Ahmed Badr Neil Sadler Study Team Ahmed Badr Rasha Hassan, Lead Researcher Enas Hamdy Enas Hamdy, Researcher Ahmed Badr, Researcher Photo: Ahmed Jabber Acknowledgments This report is the result of partnerships between various entities, including international organizations, independent initiatives and civil society organizations. It is a collaborative and coordinated endeavor our warmest acknowledgements to all of the groups, organizations, and individuals who offered staff provided us with valuable comments and support during the development and the implementation of research, namely Dr Matthew Smith, Dr Adel El Zaeem, Dr Khaled El-Foraty, Dr Laurent Elder, Dr. Naser Faruqui, and Ms Jihan Saeed. This project was implemented under the auspices of the Youth and Development Consultancy Institute (Etijah) and special thanks go to Mr Hisham El Rouby, Director, Mr for their efforts and the support they have provided. The research advisory group have been a major asset in the development of the research protocol Maia Sieverding, and Dr Faten Abdel Fattah. Further, we would like to thank Dr Muhammed Nour who shouldered the responsibility of identifying a representative sample of the target population. -

Of the Anthophora-Species of Egypt
Prof. Dr. H. PRIESNER A REVIEW OF THE ANTHOPHORA-SPECIES OF EGYPT [Hymenoptera : Apidae] A HEVIEW OF THE ANTHOPHORA-SPECIES OF EGYPT (Hymenoptera : Apidae] by Prof. Dr. H. PRIESNER INTRODUCTION Owing to the difficulties I encountered in trying to identify the Egyptian material of Anthophora in the collections of the Cairo and Ain Shams Universities, I had to penetrate more deeply into this matter, especially when I found that quite a number of species of the local collections were no doubt incorrectly named. With the progress of science, in our particular case with the improvements achieved by finding new distinctive characters and especially in the better relative evaluation of those already known, gained by experience and eye training, there lies upon us the bitter task to criticise and correct our late authorities on this subject who actually did all the spade work that enabled us to start on a considerable higher level of knowledge than that having been at their disposal. Apart from the relatively excellent work of KLUG (1845) and a number of species described by SPINOLA and - much later - GRIBODO, most of the taxonomic work on Anthophora of this country was done by H. FRIESE and A. ALFKEN, their work having been mainly based upon the Apid collections of our late A. ANDRES, while my late friend ALFKEN had also examined specimens he received from the Ministry of Agriculture, Mr. A. ALFIERI and the author. ALFKEN had, obviously with the intention of avoiding the creation of synonyms, identified a number of Egyptian species with such of Asiatic origin. -

Egypt As You Have Never Seen Before
EGYPT AS YOU HAVE NEVER SEEN BEFORE DAY 010101 : Arrival at Cairo airport, meet and assist through formalities transfer to the hotel for overnight. DAYDAY02020202 : Breakfast, start our tour by visiting the palace of Mohamed Ali the ruler who modernized Egypt, built himself a palace in Shubra In the 17th century and extended Shubra Street all the way to his palace in 1808. It was recorded that the street had fig and date trees on its sides. We proceed to MATARYA where the Holy Mother washed clothes of her son, and from the water God caused a fragrant plant to grow, "Balsam”. There is also St. Mary's tree to visit. Head towards to visit Ibn Toulon mosque one of the famous Cairo mosques and the focal point of the Tulunid capital that lasted only 26 years. It was the third congregational mosque to be built in what is now greater Cairo, and at approximately 26,318 square meters in size, is the third largest mosque in the world. Back to the hotel, dinner and overnight. palace of Mohamed Ali The Palace of Muhammad Ali locates in Shubra El-Kheima next to the Faculty of Agriculture there. For visiting this palace, one can easily take the metro rather than a taxi. The palace was established by Mohammed Ali in the early years of assuming the power but its construction was completed many years later and was the first building to be built in that agricultural area. After that, this area was occupied with a lot of luxuriously decorated and gracefully designed palaces for established by the prominent noblemen at that time including the palace of Qasr El- Nil, Qasr El-Dubara and others that disappeared now. -
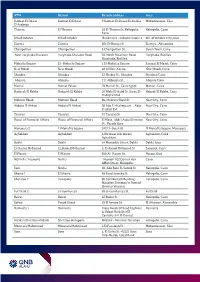
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St.