12 17 December 2008
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skin Microbiota: a Source of Disease Or Defence? A.L
FROM BENCH TO BEDSIDE DOI 10.1111/j.1365-2133.2008.08437.x Skin microbiota: a source of disease or defence? A.L. Cogen,* V. Nizetৠand R.L. Gallo à Departments of *Bioengineering, Medicine, Division of Dermatology and àPediatrics, School of Medicine, and §Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, CA, U.S.A. Summary Correspondence Microbes found on the skin are usually regarded as pathogens, potential patho- Richard L. Gallo. gens or innocuous symbiotic organisms. Advances in microbiology and immu- E-mail: [email protected] nology are revising our understanding of the molecular mechanisms of microbial virulence and the specific events involved in the host–microbe interaction. Cur- Accepted for publication 30 September 2007 rent data contradict some historical classifications of cutaneous microbiota and suggest that these organisms may protect the host, defining them not as simple Key words symbiotic microbes but rather as mutualistic. This review will summarize current bacteria, immunity, infectious disease information on bacterial skin flora including Staphylococcus, Corynebacterium, Propioni- bacterium, Streptococcus and Pseudomonas. Specifically, the review will discuss our cur- Conflicts of interest rent understanding of the cutaneous microbiota as well as shifting paradigms in None declared. the interpretation of the roles microbes play in skin health and disease. Most scholarly reviews of skin microbiota concentrate on ence inflammatory bowel disease,2 and how lactobacilli in the understanding the population structure of the flora inhabiting intestine educate prenatal immune responses. These findings the skin, or how a subset of these microbes can become complement several studies that suggest disruption in micro- human pathogens. -

Bacterial Pseudomycetoma (Botryomycosis) in an FIV-Positive Cat
獣医臨床皮膚科 16 (2): 61–65, 2010 Case Report Bacterial Pseudomycetoma (Botryomycosis) in an FIV-positive Cat FIV陽性猫にみられた細菌性偽菌腫(ボトリオミセス症)の1例 Tae Murai1)*, Kyohei Yasuno2), Kinji Shirota2, 3) 1)Kinder-Care Veterinary Clinic, 2)Research Institute of Biosciences and 3)Laboratory of Veterinary Pathology, Azabu University 村井 妙 1)* 安野恭平 2) 代田欣二 2, 3) 1)キンダーケア動物病院,2)麻布大学附置生物科学総合研究所,3)麻布大学獣医病理学研究室 Abstract: A 2.5-year-old spayed female domestic short-haired cat presented with more than a month’s history of a thickly crusted, purulent draining lesion on the shoulder area. Prior to the lesion’s formation, the cat had been repeatedly scratching the shoulder. Histological examination of skin biopsy specimens revealed both discrete and confluent pyogranulomas with fibroplasias and characteristic granules composed of gram-positive cocci surrounded by an eosinophilic amorphous substance. The histopathological findings were consistent with bacterial pseudomycetoma. The skin lesion was successfully treated with systemic and topical administration of antibiotics for about 4 months. However, occasional scratching continued, leading to the development of a small erosion in the same area. The present cat was antigen-positive for feline immunodeficiency virus, and it showed symptoms potentially caused by feline immunodeficiency syndrome. Decreased immune competence might contribute to the pathogenesis of bacterial pseudomycetoma. Key words: bacterial pseudomycetoma, botryomycosis, cat 要 約:2.5歳齢,避妊済みの雑種猫が,頚背部に著しく厚い痂疲を伴う排膿性皮疹を呈し来院した。 病変は当初激しいそう痒から始まり,やがて掻爬部位に一致して瘻孔の形成を認めた。病変部の病 -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Pseudomonas Skin Infection Clinical Features, Epidemiology, and Management
Am J Clin Dermatol 2011; 12 (3): 157-169 THERAPY IN PRACTICE 1175-0561/11/0003-0157/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. Pseudomonas Skin Infection Clinical Features, Epidemiology, and Management Douglas C. Wu,1 Wilson W. Chan,2 Andrei I. Metelitsa,1 Loretta Fiorillo1 and Andrew N. Lin1 1 Division of Dermatology, University of Alberta, Edmonton, Alberta, Canada 2 Department of Laboratory Medicine, Medical Microbiology, University of Alberta, Edmonton, Alberta, Canada Contents Abstract........................................................................................................... 158 1. Introduction . 158 1.1 Microbiology . 158 1.2 Pathogenesis . 158 1.3 Epidemiology: The Rise of Pseudomonas aeruginosa ............................................................. 158 2. Cutaneous Manifestations of P. aeruginosa Infection. 159 2.1 Primary P. aeruginosa Infections of the Skin . 159 2.1.1 Green Nail Syndrome. 159 2.1.2 Interdigital Infections . 159 2.1.3 Folliculitis . 159 2.1.4 Infections of the Ear . 160 2.2 P. aeruginosa Bacteremia . 160 2.2.1 Subcutaneous Nodules as a Sign of P. aeruginosa Bacteremia . 161 2.2.2 Ecthyma Gangrenosum . 161 2.2.3 Severe Skin and Soft Tissue Infection (SSTI): Gangrenous Cellulitis and Necrotizing Fasciitis. 161 2.2.4 Burn Wounds . 162 2.2.5 AIDS................................................................................................. 162 2.3 Other Cutaneous Manifestations . 162 3. Antimicrobial Therapy: General Principles . 163 3.1 The Development of Antibacterial Resistance . 163 3.2 Anti-Pseudomonal Agents . 163 3.3 Monotherapy versus Combination Therapy . 164 4. Antimicrobial Therapy: Specific Syndromes . 164 4.1 Primary P. aeruginosa Infections of the Skin . 164 4.1.1 Green Nail Syndrome. 164 4.1.2 Interdigital Infections . 165 4.1.3 Folliculitis . -

Dermatology in the ER
DRUG ERUPTIONS and OTHER DISORDERS Lloyd J. Cleaver D.O. , F.A.O.C.D, F.A.A.D. Professor of Dermatology ATSU-Kirksville College of Osteopathic Medicine INTERNAL MEDICINE BOARD REVIEW COURSE I Disclosures No Relevant Financial Relationships DRUG ERUPTIONS Drug Reactions 3 things you need to know 1. Type of drug reaction 2. Statistics What drugs are most likely to cause that type of reaction? 3. Timing How long after the drug was started did the reaction begin? Clinical Pearls Drug eruptions are extremely common Tend to be generalized/symmetric Maculopapular/morbilliform are most common Best Intervention: Stop the Drug! Do not dose reduce Completely remove the exposure How to spot the culprit? Drug started within days to a week prior to rash Can be difficult and take time Tip: can generally exclude all drugs started after onset of rash Drug eruptions can continue for 1-2 weeks after stopping culprit drug LITT’s drug eruption database Drug Eruptions Skin is one of the most common targets for drug reactions Antibiotics and anticonvulsants are most common 1-5% of patients 2% of all drug eruptions are “serious” TEN, DRESS More common in adult females and boys < 3 y/o Not all drugs cause eruptions at same rate: Aminopenicillins: 1.2-8% of exposures TMP-SMX: 2.8-3.7% NSAIDs: 1 in 200 Lamotrigine: 10% Drug Eruptions Three basic rules 1. Stop any unnecessary medications 2. Ask about non-prescription medications Eye drops, suppositories, implants, injections, patches, vitamin and health supplements, friend’s medications -

Wednesday Slide Conference 2008-2009
PROCEEDINGS DEPARTMENT OF VETERINARY PATHOLOGY WEDNESDAY SLIDE CONFERENCE 2008-2009 ARMED FORCES INSTITUTE OF PATHOLOGY WASHINGTON, D.C. 20306-6000 2009 ML2009 Armed Forces Institute of Pathology Department of Veterinary Pathology WEDNESDAY SLIDE CONFERENCE 2008-2009 100 Cases 100 Histopathology Slides 249 Images PROCEEDINGS PREPARED BY: Todd Bell, DVM Chief Editor: Todd O. Johnson, DVM, Diplomate ACVP Copy Editor: Sean Hahn Layout and Copy Editor: Fran Card WSC Online Management and Design Scott Shaffer ARMED FORCES INSTITUTE OF PATHOLOGY Washington, D.C. 20306-6000 2009 ML2009 i PREFACE The Armed Forces Institute of Pathology, Department of Veterinary Pathology has conducted a weekly slide conference during the resident training year since 12 November 1953. This ever- changing educational endeavor has evolved into the annual Wednesday Slide Conference program in which cases are presented on 25 Wednesdays throughout the academic year and distributed to 135 contributing military and civilian institutions from around the world. Many of these institutions provide structured veterinary pathology resident training programs. During the course of the training year, histopathology slides, digital images, and histories from selected cases are distributed to the participating institutions and to the Department of Veterinary Pathology at the AFIP. Following the conferences, the case diagnoses, comments, and reference listings are posted online to all participants. This study set has been assembled in an effort to make Wednesday Slide Conference materials available to a wider circle of interested pathologists and scientists, and to further the education of veterinary pathologists and residents-in-training. The number of histopathology slides that can be reproduced from smaller lesions requires us to limit the number of participating institutions. -
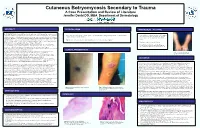
Cutaneous Botryomycosis Secondary to Trauma: a Case Presentation
Cutaneous Botryomycosis Secondary to Trauma A Case Presentation and Review of Literature Jennifer David DO, MBA Department of Dermatology ABSTRACT PHYSICAL EXAM MANAGEMENT / OUTCOME CASE DESCRIPTION: A 52-year-old Caucasian male with a past medical history of hypertension presented • Tissue cultures were obtained and showed to be negative to our clinic complaining of growths on bilateral forearms that developed two months prior. The lesion on his • Left forearm: 4cm pink exophytic vegetative plaque with central ulceration, crusting and background of excoriations and pink for acid-fast mycobacteria and fungal/yeast elements right forearm began as small pink papules that grew over the course of a few weeks, ulcerated and developed a atrophic scars on left forearm. (Figure 1) however did grow heavy amounts of Staphylococcus crusted scab. His main concern was regarding the growing lesion on the right arm. He admitted to occasional aureus. mild pruritus but denied any associated pain, tenderness or burning sensation of involved skin. Of note, he • Right forearm : scattered excoriations, pink/tan papules and a 1 cm crusted ulceration (Figure 2) worked as a HVAC (heating, ventilation and air conditioning) repairman with a history of repeated trauma to • Deep shave biopsy of lesion on left forearm his forearms due to reaching through confined spaces of larger industrial units. On physical exam patient presented with 4cm pink exophytic vegetative plaque with central ulceration, • Patient was treated with oral cephalexin 500mg and crusting and background of excoriations and pink atrophic scars on left forearm. (Figure 1) On the right topical mupirocin ointment twice daily for two weeks. -

Dermatological Indications of Disease - Part II This Patient on Dialysis Is Showing: A
“Cutaneous Manifestations of Disease” ACOI - Las Vegas FR Darrow, DO, MACOI Burrell College of Osteopathic Medicine This 56 year old man has a history of headaches, jaw claudication and recent onset of blindness in his left eye. Sed rate is 110. He has: A. Ergot poisoning. B. Cholesterol emboli. C. Temporal arteritis. D. Scleroderma. E. Mucormycosis. Varicella associated. GCA complex = Cranial arteritis; Aortic arch syndrome; Fever/wasting syndrome (FUO); Polymyalgia rheumatica. This patient missed his vaccine due at age: A. 45 B. 50 C. 55 D. 60 E. 65 He must see a (an): A. neurologist. B. opthalmologist. C. cardiologist. D. gastroenterologist. E. surgeon. Medscape This 60 y/o male patient would most likely have which of the following as a pathogen? A. Pseudomonas B. Group B streptococcus* C. Listeria D. Pneumococcus E. Staphylococcus epidermidis This skin condition, erysipelas, may rarely lead to septicemia, thrombophlebitis, septic arthritis, osteomyelitis, and endocarditis. Involves the lymphatics with scarring and chronic lymphedema. *more likely pyogenes/beta hemolytic Streptococcus This patient is susceptible to: A. psoriasis. B. rheumatic fever. C. vasculitis. D. Celiac disease E. membranoproliferative glomerulonephritis. Also susceptible to PSGN and scarlet fever and reactive arthritis. Culture if MRSA suspected. This patient has antithyroid antibodies. This is: • A. alopecia areata. • B. psoriasis. • C. tinea. • D. lichen planus. • E. syphilis. Search for Hashimoto’s or Addison’s or other B8, Q2, Q3, DRB1, DR3, DR4, DR8 diseases. This patient who works in the electronics industry presents with paresthesias, abdominal pain, fingernail changes, and the below findings. He may well have poisoning from : A. lead. B. -

Research Article
z Available online at http://www.journalcra.com INTERNATIONAL JOURNAL OF CURRENT RESEARCH International Journal of Current Research Vol. 8, Issue, 06, pp.33640-33642, June, 2016 ISSN: 0975-833X RESEARCH ARTICLE BOTRYOMYCOSIS IN A YOUNG MALE: A CASE REPORT AND REVIEW OF LITERATURE Dr. Satish Deshmukh, *Dr. Divish Saxena, Dr. Rajiv Sonarkar and Dr. Rahul Saboo Department of Surgery, NKP Salve Institute of Medical Sciences, Digdoh Hills, Nagpur ARTICLE INFO ABSTRACT Article History: Botryomycosis also known as bacterial pseudo mycosis is a rare chronic granulomatous bacterial th infection that affects the skin and sometimes the viscera. The lesions are characterized by tumefaction, Received 14 March, 2016 Received in revised form deformity, multiple sinus formation and fistulous tract with deep abscess and ulcerated area of skin. 28th April, 2016 Common organisms include staphylococcus, pseudomonas, E.coli, proteus and streptococcus species. Accepted 15th May, 2016 Botryomycosis has been known to affect humans as well as other mammals. We report a case of Published online 30th June, 2016 anaerobic Botryomycosis occurring in a young male patient who presented with atypical skin lesions at two different anatomical sites with a previous history of trauma. Complete excision of the lesion Key words: with primary closure of skin was done with excellent results. The final histopathological report of excised lesion was suggestive of botryomycosis with Hoeppli- Splendore reaction. We report this case Botryomycosis, as only few cases occurring in human beings are described in literature. Chronic granulomatous infection, Pseudo mycosis, Hoeppli- Splendore phenomenon. Copyright©2016, Dr. Satish Deshmukh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

A Case of CD20 Positive Peripheral T-Cell Lymphoma: Not Otherwise Specified Masquerading As Botryomycosis
International Journal of Research in Dermatology Potlapati A et al. Int J Res Dermatol. 2019 Nov;5(4):910-913 http://www.ijord.com DOI: http://dx.doi.org/10.18203/issn.2455-4529.IntJResDermatol20194695 Case Report A case of CD20 positive peripheral T-cell lymphoma: not otherwise specified masquerading as botryomycosis Amruthavalli Potlapati, Neethu Mary George*, Narendra Gangaiah, Veena Thimmappa Department of Dermatology, Sri Siddhartha Medical College, Tumkur, Karnataka, India Received: 02 June 2019 Accepted: 26 July 2019 *Correspondence: Dr. Neethu Mary George, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Peripheral T-cell lymphoma (PTCL), a type of non-Hodgkin’s lymphoma, is an uncommon malignancy frequently involving the skin either as primary or secondary manifestation of the disease. Lymphomas may occasionally masquerade as infectious diseases especially when it is not associated with significant lymphadenopathy. Botryomycosis is a chronic granulomatous suppurative bacterial infection, predominantly caused by Staphylococcus aureus, which is seen on trauma prone sites. Herein we present a case of a 33 years old male who presented to us with multiple rapidly growing friable masses on the chest wall of six weeks duration which clinically pointed towards botryomycosis. Lack of response to treatment with intravenous antibiotics and absence of grains and granuloma in the biopsy specimen made us further proceed for immune-histochemical evaluation which confirmed a rare type of CD20 positive PTCL. -

214 Diagnosis of Infectious Diseases in Surgical and Cytopathology with Microbiology Recommendations
214 Diagnosis of Infectious Diseases in Surgical and Cytopathology with Microbiology Recommendations Sherri Yong MD Eva Wojcik MD 2011 Annual Meeting – Las Vegas, NV AMERICAN SOCIETY FOR CLINICAL PATHOLOGY 33 W. Monroe, Ste. 1600 Chicago, IL 60603 214 Diagnosis of Infectious Diseases in Surgical and Cytopathology with Microbiology Recommendations This session will cover the identification of viral, bacterial, fungal and parasitic infectious disease agents in surgical pathology and cytopathology specimens with recommendations for microbiological studies. This session will address commonly encountered infectious diseases as well as emerging and re-emerging infectious disease agents. Special attention to common infectious agents that might be encountered from global traveling will also be addressed. Participants will be encouraged to share their experiences and diagnostic practice. • Participants will be able to evaluate surgical and cytology specimens for the identification of infectious disease agents. • Participants will be able to decide on microbiological studies important for the identification of infectious disease agents and /or communicating such studies to clinicians. • Participants with establish a working knowledge of emerging infectious diseases and infectious diseases common to travel abroad. FACULTY: Sherri Yong MD Eva Wojcik MD Entire Pathology Team Global Pathology Global Pathology 2.0 CME/CMLE Credits Accreditation Statement: The American Society for Clinical Pathology (ASCP) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education (CME) for physicians. This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME). Credit Designation: The ASCP designates this enduring material for a maximum of 2 AMA PRA Category 1 Credits™. -

Infectious Diseases of the Horse
INFECTIOUS DISEASES of the Diagnosis, pathology, HORSE management, and public health J. H. van der Kolk DVM, PhD, dipl ECEIM Department of Equine Sciences–Medicine Faculty of Veterinary Medicine Utrecht University, the Netherlands E. J. B. Veldhuis Kroeze DVM, dipl ECVP Department of Pathobiology Faculty of Veterinary Medicine Utrecht University, the Netherlands MANSON PUBLISHING / THE VETERINARY PRESS Copyright © 2013 Manson Publishing Ltd ISBN: 978-1-84076-165-8 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means without the written permission of the copyright holder or in accordance with the provisions of the Copyright Act 1956 (as amended), or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 33–34 Alfred Place, London WC1E 7DP, UK. Any person who does any unauthorized act in relation to this publication may be liable to criminal prosecution and civil claims for damages. A CIP catalogue record for this book is available from the British Library. For full details of all Manson Publishing Ltd titles please write to: Manson Publishing Ltd, 73 Corringham Road, London NW11 7DL, UK. Tel: +44(0)20 8905 5150 Fax: +44(0)20 8201 9233 Email: [email protected] Website: www.mansonpublishing.com Commissioning editor: Jill Northcott Project manager: Kate Nardoni Copy editor: Ruth Maxwell Layout: DiacriTech, Chennai, India Colour reproduction: Tenon & Polert Colour Scanning Ltd, Hong Kong Printed by: Grafos SA, Barcelona, Spain CONTENTS Introduction 5 Actinomyces spp. 90 Abbreviations 6 Dermatophilus congolensis: ‘rain scald’ or streptotrichosis 92 Chapter 1 Corynebacterium pseudotuberculosis: ‘pigeon fever’ 94 Bacterial diseases 9 Mycobacterium spp.: tuberculosis 97 Nocardia spp.