WSC 13-14 Conf 7 Layout
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Pancreatitis, Non-Alcoholic Fatty Pancreas Disease, and Pancreatic Cancer
JOP. J Pancreas (Online) 2017 Sep 29; 18(5):365-368. REVIEW ARTICLE The Burden of Systemic Adiposity on Pancreatic Disease: Acute Pancreatitis, Non-Alcoholic Fatty Pancreas Disease, and Pancreatic Cancer Ahmad Malli, Feng Li, Darwin L Conwell, Zobeida Cruz-Monserrate, Hisham Hussan, Somashekar G Krishna Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA ABSTRACT Obesity is a global epidemic as recognized by the World Health Organization. Obesity and its related comorbid conditions were recognized to have an important role in a multitude of acute, chronic, and critical illnesses including acute pancreatitis, nonalcoholic fatty pancreas disease, and pancreatic cancer. This review summarizes the impact of adiposity on a spectrum of pancreatic diseases. INTRODUCTION and even higher mortality in the setting of AP based on multiple reports [8, 9, 10, 11, 12]. Despite the rising incidence Obesity is a global epidemic as recognized by the World of AP over the past two decades, there has been a decrease Health Organization [1]. One third of the world’s population in its overall mortality rate without any obvious decrement is either overweight or obese, and it has doubled over the in the mortality rate among patients with concomitant AP past two decades with an alarming 70% increase in the and morbid obesity [12, 13]. Several prediction models and prevalence of morbid obesity from year 2000 to 2010 [2, risk scores were proposed to anticipate the severity and 3, 4]. Obesity and its related comorbid conditions were prognosis of patient with AP; however, their clinical utility recognized to have an important role in a multitude of is variable, not completely understood, and didn’t take acute, chronic, and critical pancreatic illnesses including obesity as a major contributor into consideration despite the acute pancreatitis, non-alcoholic fatty pancreas disease, aforementioned association [14]. -

Chapter 1 Cellular Reaction to Injury 3
Schneider_CH01-001-016.qxd 5/1/08 10:52 AM Page 1 chapter Cellular Reaction 1 to Injury I. ADAPTATION TO ENVIRONMENTAL STRESS A. Hypertrophy 1. Hypertrophy is an increase in the size of an organ or tissue due to an increase in the size of cells. 2. Other characteristics include an increase in protein synthesis and an increase in the size or number of intracellular organelles. 3. A cellular adaptation to increased workload results in hypertrophy, as exemplified by the increase in skeletal muscle mass associated with exercise and the enlargement of the left ventricle in hypertensive heart disease. B. Hyperplasia 1. Hyperplasia is an increase in the size of an organ or tissue caused by an increase in the number of cells. 2. It is exemplified by glandular proliferation in the breast during pregnancy. 3. In some cases, hyperplasia occurs together with hypertrophy. During pregnancy, uterine enlargement is caused by both hypertrophy and hyperplasia of the smooth muscle cells in the uterus. C. Aplasia 1. Aplasia is a failure of cell production. 2. During fetal development, aplasia results in agenesis, or absence of an organ due to failure of production. 3. Later in life, it can be caused by permanent loss of precursor cells in proliferative tissues, such as the bone marrow. D. Hypoplasia 1. Hypoplasia is a decrease in cell production that is less extreme than in aplasia. 2. It is seen in the partial lack of growth and maturation of gonadal structures in Turner syndrome and Klinefelter syndrome. E. Atrophy 1. Atrophy is a decrease in the size of an organ or tissue and results from a decrease in the mass of preexisting cells (Figure 1-1). -

Non-Cancerous Breast Conditions Fibrosis and Simple Cysts in The
cancer.org | 1.800.227.2345 Non-cancerous Breast Conditions ● Fibrosis and Simple Cysts ● Ductal or Lobular Hyperplasia ● Lobular Carcinoma in Situ (LCIS) ● Adenosis ● Fibroadenomas ● Phyllodes Tumors ● Intraductal Papillomas ● Granular Cell Tumors ● Fat Necrosis and Oil Cysts ● Mastitis ● Duct Ectasia ● Other Non-cancerous Breast Conditions Fibrosis and Simple Cysts in the Breast Many breast lumps turn out to be caused by fibrosis and/or cysts, which are non- cancerous (benign) changes in breast tissue that many women get at some time in their lives. These changes are sometimes called fibrocystic changes, and used to be called fibrocystic disease. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Fibrosis and cysts are most common in women of child-bearing age, but they can affect women of any age. They may be found in different parts of the breast and in both breasts at the same time. Fibrosis Fibrosis refers to a large amount of fibrous tissue, the same tissue that ligaments and scar tissue are made of. Areas of fibrosis feel rubbery, firm, or hard to the touch. Cysts Cysts are fluid-filled, round or oval sacs within the breasts. They are often felt as a round, movable lump, which might also be tender to the touch. They are most often found in women in their 40s, but they can occur in women of any age. Monthly hormone changes often cause cysts to get bigger and become painful and sometimes more noticeable just before the menstrual period. Cysts begin when fluid starts to build up inside the breast glands. Microcysts (tiny, microscopic cysts) are too small to feel and are found only when tissue is looked at under a microscope. -

Official Proceedings
Scientific Session Awards Abstracts presented at the Society’s annual meeting will be considered for the following awards: • The George Peters Award recognizes the best presentation by a breast fellow. In addition to a plaque, the winner receives $1,000. The winner is selected by the Society’s Publications Committee. The award was established in 2004 by the Society to honor Dr. George N. Peters, who was instrumental in bringing together the Susan G. Komen Breast Cancer Foundation, The American Society of Breast Surgeons, the American Society of Breast Disease, and the Society of Surgical Oncology to develop educational objectives for breast fellowships. The educational objectives were first used to award Komen Interdisciplinary Breast Fellowships. Subsequently the curriculum was used for the breast fellowship credentialing process that has led to the development of a nationwide matching program for breast fellowships. • The Scientific Presentation Award recognizes an outstanding presentation by a resident, fellow, or trainee. The winner of this award is also determined by the Publications Committee. In addition to a plaque, the winner receives $500. • All presenters are eligible for the Scientific Impact Award. The recipient of the award, selected by audience vote, is honored with a plaque. All awards are supported by The American Society of Breast Surgeons Foundation. The American Society of Breast Surgeons 2 2017 Official Proceedings Publications Committee Chair Judy C. Boughey, MD Members Charles Balch, MD Sarah Blair, MD Katherina Zabicki Calvillo, MD Suzanne Brooks Coopey, MD Emilia Diego, MD Jill Dietz, MD Mahmoud El-Tamer, MD Mehra Golshan, MD E. Shelley Hwang, MD Susan Kesmodel, MD Brigid Killelea, MD Michael Koretz, MD Henry Kuerer, MD, PhD Swati A. -
Fat Necrosis
Fat necrosis This leaflet tells you about fat necrosis. It explains what fat necrosis is, how it’s diagnosed and what will happen if it needs to be followed up or treated. Benign breast conditions information provided by Breast Cancer Care Breast Cancer Care doesn’t just support people when they’ve been diagnosed with breast cancer. We also highlight the importance of early detection and provide up-to-date, expert information on breast conditions and breast health. If you have a question about breast health or breast cancer you can call us free on 0808 800 6000 or visit breastcancercare.org.uk We hope you find this information useful. If you’d like to help ensure we’re there for other people when they need us visit breastcancercare.org.uk/donate Central Office Breast Cancer Care Chester House 1–3 Brixton Road London SW9 6DE Phone: 0345 092 0800 Email: [email protected] Call our Helpline on 0808 800 6000 What is fat necrosis? Fat necrosis is a benign (not cancer) condition and does not increase your risk of developing breast cancer. It can occur anywhere in the breast and can affect women of any age. Men can also get fat necrosis, but this is very rare. Breasts are made up of lobules (milk-producing glands) and ducts (tubes that carry milk to the nipple). These are surrounded by glandular, fibrous and fatty tissue. Sometimes a lump can form if an area of the fatty breast tissue is damaged. This is called fat necrosis (necrosis is a medical term used to describe damaged or dead tissue). -

CELL INJURY Lecture 3
CELL INJURY Lecture 3 َ{ عَّلَم ْ ِٕ النَسَانَمَا لْم يَْعَلْم } Objectives: ● Understand the causes of and pathologic changes occurring in fatty change (steatosis), accumulations of exogenous and endogenous pigments (carbon, silica, iron, melanin, bilirubin and lipofuscin). ● Understand the causes of and differences between dystrophic and metastatic calcifications. Color Index: Girl’s Slides Important Male’s Notes Female’s Notes 1 Extra information Substance accumulate inside the cell in large Intracellular amounts and cause problems in the cell and the Accumulation: organ it’s called intracellular accumulation. The substance may accumulate in either the cytoplasm or nucleus. And can be: An abnormal A substance that’s substance not present A pigment: it can be always present in normal in the cell normally. an endogenous or an cell but has accumulated exogenous. excess e.g. water, lipids, glycogen, protein, carbohydrates It can be: Endogenous (from Exogenous (from outside inside the body) e.g. a the body) e.g. a mineral or product of abnormal component of bacteria. synthesis or metabolism. ExamplesExamples of of substances substance thatthat accumulateaccumulate in in excess excess in in the the cell: cell: A) water: abnormal accumulation of water in the cells is called hydropic change (cellular swelling). It’s an early sign of cellular degeneration in response to injury (note: it’s due to the failure of energy-dependent ion pump on the plasma membrane ⟶ leading to loss of normal ion & fluid homeostasis). B) Lipids: all major classes of lipids can accumulate in cells: • Accumulation of triglycerides ⟶ steatosis (Fatty change) • Accumulation of cholesterol ⟶ seen in the Atherosclerosis. -
Difference Between Apoptosis and Necrosis
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/315763939 Difference Between Apoptosis and Necrosis Article · April 2017 CITATIONS READS 0 22,403 1 author: Lakna Panawala Difference Between, Sydney, Australia 246 PUBLICATIONS 16 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Biochemistry View project Evolution View project All content following this page was uploaded by Lakna Panawala on 04 April 2017. The user has requested enhancement of the downloaded file. 4/4/2017 Difference Between Apoptosis and Necrosis | Definition, Process, Function, Comparison EXPLORE Type here to search... EXPLORE Home » Science » Biology » Cell Biology » Difference Between Apoptosis and Necrosis Difference Between Apoptosis and Necrosis April 4, 2017 • by Lakna • 7 min read 0 Stunning images of cells Main Difference – Apoptosis vs AAT Bioquest Discover how scientists use immunofluorescence to Necrosis capture beautiful cell images! Go to aatbio.com/science/biology Apoptosis and necrosis are two mechanisms involved in the Start Download View PDF cell death in multicellular organisms. Apoptosis is considered Convert From Doc to PDF, PDF to Doc Simply With The Free Online App! Go to download.fromdoctopdf.com as a naturally occurring physiological process whereas Instant smartness necrosis is a pathological process, which is caused by external Smarten up and impress the world. Access 50+ agents like toxins, trauma, and infections. Apoptosis is a courses developed by experts. Go to pages.mobileacademy.com highly regulated, timely process whereas the necrosis is an Structural Biology unregulated, random process. Inflammation and tissue damage Get Closer to Your Samples With TEM Imaging Tools Go to gatan.com/LifeScience are observed in necrosis. -

Infected Retroperitoneal Fat Necrosis After Laparoscopic Partial Nephrectomy D
Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2018 Infected retroperitoneal fat necrosis after laparoscopic partial nephrectomy D. Nethala Northwell Health W. J. Wu Northwell Health P. K. Mistry Northwell Health L. Richstone Zucker School of Medicine at Hofstra/Northwell Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Urology Commons Recommended Citation Nethala D, Wu WJ, Mistry PK, Richstone L. Infected retroperitoneal fat necrosis after laparoscopic partial nephrectomy. 2018 Jan 01; 17():Article 4212 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/4212. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. For more information, please contact [email protected]. Urology Case Reports 17 (2018) 103e105 Contents lists available at ScienceDirect Urology Case Reports journal homepage: www.elsevier.com/locate/eucr Oncology Infected retroperitoneal fat necrosis after laparoscopic partial nephrectomy * Daniel Nethala , Wayland J. Wu, Preeya K. Mistry, Lee Richstone Department of Urology, Smith Institute for Urology, Hofstra Northwell School of Medicine, Lake Success NY, USA article info Article history: renal mass (Fig. 1). Intraoperatively, the surgery was notable for Received 14 December 2017 entrance into the collecting system to completely resect the tumor. Accepted 17 January 2018 The collecting system was closed during renorrhaphy and a drain Available online 3 February 2018 was left in place. His postoperative course was uneventful; the drain was removed after output was minimal and he was subse- Keywords: quently discharged on postoperative day (POD) four. -

2014 Slide Library Case Summary Questions & Answers With
2014 Slide Library Case Summary Questions & Answers with Discussions 51st Annual Meeting November 6-9, 2014 Chicago Hilton & Towers Chicago, Illinois The American Society of Dermatopathology ARTHUR K. BALIN, MD, PhD, FASDP FCAP, FASCP, FACP, FAAD, FACMMSCO, FASDS, FAACS, FASLMS, FRSM, AGSF, FGSA, FACN, FAAA, FNACB, FFRBM, FMMS, FPCP ASDP REFERENCE SLIDE LIBRARY November 2014 Dear Fellows of the American Society of Dermatopathology, The American Society of Dermatopathology would like to invite you to submit slides to the Reference Slide Library. At this time there are over 9300 slides in the library. The collection grew 2% over the past year. This collection continues to grow from member’s generous contributions over the years. The slides are appreciated and are here for you to view at the Sally Balin Medical Center. Below are the directions for submission. Submission requirements for the American Society of Dermatopathology Reference Slide Library: 1. One H & E slide for each case (if available) 2. Site of biopsy 3. Pathologic diagnosis Not required, but additional information to include: 1. Microscopic description of the slide illustrating the salient diagnostic points 2. Clinical history and pertinent laboratory data, if known 3. Specific stain, if helpful 4. Clinical photograph 5. Additional note, reference or comment of teaching value Teaching sets or collections of conditions are especially useful. In addition, infrequently seen conditions are continually desired. Even a single case is helpful. Usually, the written submission requirement can be fulfilled by enclosing a copy of the pathology report prepared for diagnosis of the submitted case. As a guideline, please contribute conditions seen with a frequency of less than 1 in 100 specimens. -
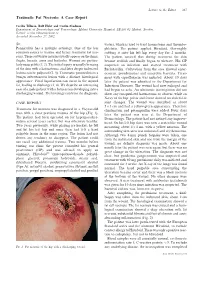
Traumatic Fat Necrosis: a Case Report
Letters to the Editor 227 Traumatic Fat Necrosis: A Case Report Cecilia Tillman, Rolf Holst and Cecilia Svedman Department of Dermatology and Venereology, Malmo¨ University Hospital, SE-205 02 Malmo¨, Sweden. E-mail: [email protected] Accepted November 27, 2002. Sir, water), which is used to treat haematoma and thrombo- Panniculitis has a multiple aetiology. One of the less phlebitis. The patient applied Hirudoid, thoroughly common causes is trauma and hence traumatic fat nec- rubbing it onto his left hip every day for 2 months. rosis. These soft tissue injuries usually appear on the shins, The patient noticed that during treatment the skin thighs, breasts, arms and buttocks. Women are particu- became reddish and finally began to ulcerate. His GP larly susceptible (1, 2). The initial injury is usually bruising suspected an infection and started treatment with of the skin with a haematoma, and later deeper indurated flucloxacillin. Cultivation from the area showed enter- lesions can be palpated (2, 3). Traumatic panniculitis is a ococcus, pseudomonas and anaerobic bacteria. Treat- benign subcutaneous lesion with a distinct histological ment with ciprofloxacin was initiated. About 10 days appearance. Focal liquefaction can occur in the injured later the patient was admitted to the Department for fat, leading to discharge (2, 4). We describe an interesting Infectious Diseases. The wound had now enlarged and case of a male patient with a fat necrosis developing into a had begun to ache. An ultrasonic investigation did not discharging wound. The histology confirms the diagnosis. show any encapsulated haematoma or abscess, while an X-ray of the hip, pelvis and femur showed no skeletal or CASE REPORT joint changes. -

Rapidly Fatal Necrotizing Fasciitis: Report of Three Cases
Rom J Leg Med [19] 95-100 [2011] DOI: 10.4323/rjlm.2011.95 © 2011 Romanian Society of Legal Medicine Rapidly fatal necrotizing fasciitis: report of three cases Karadzić Radovan1*, Goran Ilic1, Aleksandra Antovic1, Lidija Kostić Banovic1, Milic Miroslav1, Dinic Marina2 _________________________________________________________________________________________ Abstract: Necrotizing fasciitis is a rare but life-threatening soft tissue infection characterized by necrotizing process of the subcutaneous tissues and fascial planes, with resulting skin gangrene and systemic toxicity. We describe three fatal cases of clinically undiagnosed necrotizing fasciitis caused by Streptococcus pyogenes. In all cases, the evolution of disease was fulminant, and all patients were treated with non-steroidal anti-inflammatory drugs. Recently have been reported an association between using of this drugs with an increased incidence of fulminant evolution of necrotizing facciitis. The paper also highlighted the correlation between the histopathologic features of infected tissue with poor acute inflammatory response, and a high concentration of bacteria in infected tissue, which have been confirmed using Gram staining modified by Brown&Bremm. These presented cases emphasize the need for early diagnosis, because prompt clinically recognition prevents unnecessary morbidity and mortality. Key Words: Necrotizing fasciitis, Streptococcus pyogenes, Forensic ecrotizing fasciitis (NF) is agressive and destructive infection of the subcutaneous Ntissues, associated with substantial mortality and long-term morbidity. It was actually first described by Hippocrates in the 5th century BC as a complication of erysipelas [1]. NF caused by beta-hemolytic streptococci was first described by Meleney in 1924. It has been described under various synonyms, including hospital gangrene, hemolytic or acute streptococcal gangrene, Meleney’s gangrene and Fournier’s gangrene. -

Lipofilling in Breast Oncological Surgery
International Journal of Molecular Sciences Review Lipofilling in Breast Oncological Surgery: A Safe Opportunity or Risk for Cancer Recurrence? Francesca Piccotti 1 , Ilona Rybinska 2 , Elisabetta Scoccia 3, Carlo Morasso 1 , Alessandra Ricciardi 1, Lorena Signati 4, Tiziana Triulzi 2 , Fabio Corsi 3,4 and Marta Truffi 1,* 1 Laboratorio di Nanomedicina ed Imaging Molecolare, Istituti Clinici Scientifici Maugeri IRCCS, 27100 Pavia, Italy; [email protected] (F.P.); [email protected] (C.M.); [email protected] (A.R.) 2 Molecular Targeting Unit, Department of Research, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy; [email protected] (I.R.); [email protected] (T.T.) 3 Breast Unit, Surgery Department, Istituti Clinici Scientifici Maugeri IRCCS, 27100 Pavia, Italy; [email protected] (E.S.); [email protected] (F.C.) 4 Dipartimento di Scienze Biomediche e Cliniche “L. Sacco”, Università Degli Studi di Milano, 20157 Milano, Italy; [email protected] * Correspondence: marta.truffi@icsmaugeri.it; Tel.: +39-0382-592219 Abstract: Lipofilling (LF) is a largely employed technique in reconstructive and esthetic breast surgery. Over the years, it has demonstrated to be extremely useful for treatment of soft tissue defects after demolitive or conservative breast cancer surgery and different procedures have been developed to improve the survival of transplanted fat graft. The regenerative potential of LF is attributed to the multipotent stem cells found in large quantity in adipose tissue. However, a growing body of pre- clinical evidence shows that adipocytes and adipose-derived stromal cells may have pro-tumorigenic potential. Despite no clear indication from clinical studies has demonstrated an increased risk of Citation: Piccotti, F.; Rybinska, I.; Scoccia, E.; Morasso, C.; Ricciardi, A.; cancer recurrence upon LF, these observations challenge the oncologic safety of the procedure.