Swiss Biotech Report 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ski Touring in Upper Valais
Group Media Trip - Ski touring in Upper Valais Destinations: Binntal Landscape Park and Obergoms Dates: Monday 20th to Saturday 25th April 2020 (5 nights, 6 days) Participants: max. 10 journalists Highlights: Ski touring in the Binntal Landscape Park and in Obergoms. These two regions are located in and around Goms, the valley of the young river Rhône in Upper Valais. The Binn valley (Binntal) is a wild and romantic side valley of Goms also known as “the valley of hidden treasures”; Obergoms is the uppermost stretch of the Goms valley. Fitness level: 3/3 www.visitvalais.ch VALAIS/WALLIS PROMOTION IMPORTANT INFORMATION FOR THIS PRESS TRIP This press trip is for journalists looking for an introduction to ski touring. With easy routes, equipment provided and supervision by experienced guides, this trip will take you on a journey of discovery through a pristine winter landscape, where you’ll meet people who have made the mountains their place of work. Conditions for taking part in this press trip - You must be in good or very good physical shape and have the stamina and endurance to keep going for several hours a day over several days - You are able to ski on a red slope (intermediate to advanced level) Transport in Switzerland For your comfortable journey through Switzerland, Swiss Travel System AG is happy to provide you with a unique all-in-one 1st class Swiss Travel Pass. 4 advantages of your #swisstravelpass - Unlimited travel by train, bus and boat - Public transportation in more than 90 cities and towns - Includes mountain excursions: Rigi, Schilthorn, Stanserhorn and Stoos - Free admission to more than 500 museums throughout Switzerland The Grand Train Tour of Switzerland links the most scenic panoramic lines, showcasing the country’s diversity and highlights. -

Rangliste (PDF)
Veranstalter SC Obergoms Rangliste Sprint / Liste de résultat sprint Samstag, 30. November 2019 Technik frei / technique libre SC Obergoms Ulrichen Swiss Cup BKW FIS-Rennen Obergoms Samstag, 30. November 2019 Rangliste Sprint Jury Technische Daten Damen Herren TD-FIS Gérald Brandt Distanz 1280 m 1508 m TD-FIS Ass, Race Director Edi Zihlmann Start/Ziel 1343 m 1343 m Wettkampfleiter André Vogt Tiefster Punkt 1343 m 1343 m Höchster Punkt 1376 m 1376 m Höhenunterschied (HD) 33 m 33 m Technik frei Gesamtsteigung (TC) 45 m 54 m Einzelstart Intervall 15 Sekunden Höchstanstieg (MC) 31 m 31 m Code SP Quali W FIS 2834 104.92 Wetter leicht bewölkt 2 C° SP Quali M FIS 2835 98.98 Schnee kompakt, hart -1 C° SP Final W FIS 2836 SP Final M FIS 2837 SP Final W Jun 2838 Gemeldet 171 | Gestartet 164 | Rangiert 163 SP Final M Jun 2839 Rang Nr Code Name, Vorname Jg. RV / Club / Nat. Zeit R’stand FIS-Pkt Damen U20 Final 1 1 3515357 Siri WIGGER ZSV / Am Bachtel 03:08,86 2 2 3515320 Flavia LINDEGGER BSV / Davos 03:16,81 3 4 3515316 Anja LOZZA BSV / Zuoz 03:19,86 4 6 3515321 Nadja KAELIN BSV / Alpina St.Moritz 03:19,95 5 3 3515319 Anja WEBER ZSV / Am Bachtel 03:23,88 6 7 3195314 Julie PIERREL FRA 03:24,02 1/2 Final 7 9 3515299 Solene FAIVRE GJ / La Brévine 03:22,99 8 5 3515328 Emma WUTHRICH GJ / Vue-des-Alpes 03:26,21 9 8 3515375 Marina KAELIN BSV / Alpina St.Moritz 03:26,59 10 10 3195336 Felicie CHAPPAZ FRA 03:26,41 11 13 3195292 Claudie FOURNIER FRA 03:26,55 12 26 3195392 Clemence DIDIERLAURENT FRA 03:28,77 1/4 Final 13 11 3515336 Laura BUETLER ZSSV / Nordic -

Rangliste Einzelstart
Veranstalter SC Obergoms Rangliste Einzellauf/ Liste de résultat individuel Sonntag, 1. Dezember 2019 Technik klassisch/ technique classique SC Obergoms Ulrichen Swiss Cup BKW FIS-Rennen Obergoms Sonntag, 1. Dezember 2019 Rangliste Einzelstart Jury Technische Daten 7.5 km 10 km 15 km TD-FIS Gérald Brandt Distanz 3 x 2.5 km 4 x 2.5 km 6 x 2.5 km TD-FIS Ass, Race Director Edi Zihlmann Start/Ziel 1343 m 1343 m 1343 m Wettkampfleiter André Vogt Tiefster Punkt 1343 m 1343 m 1343 m Höchster Punkt 1376 m 1376 m 1376 m Technik klassisch Gesamtsteigung (TC) 276 m 368 m 552 m Einzelstart Intervall 30 Sekunden Höchstanstieg (MC) 27 m 27 m 27 m Codex 7.5 km C FIS W 2840 59.43 Wetter bewölkt 2 C° Gemeldet 161 | Gestartet 156 | Rangiert 151 15 km C FIS M 2841 31.30 Schnee kompakt, hart -1 C° 10 km C Jun M 2842 88.11 Rang Nr Code Name, Vorname Jg. RV / Club / Nat. Zeit 1 Zeit 2 Zeit 3 Zeit 4 Zeit 5 Zeit R’stand FIS-Pkt 2.5 km 5.0 km 7.5 km 10.0 km 12.5 km Damen U18 7.5 km 1 60 3515357 Siri WIGGER 2003 ZSV / Am Bachtel 07:12,3 (2) 14:53,2 (2) 22:28,5 00:00,0 0.00 2 47 3195314 Julie PIERREL 2002 FRA 07:48,6 (17) 15:55,0 (12) 24:17,5 01:49,0 64.66 3 14 3515375 Marina KAELIN 2003 BSV / Alpina St.Moritz 07:53,5 (19) 16:06,7 (16) 24:19,2 01:50,7 65.67 4 20 3195336 Felicie CHAPPAZ 2002 FRA 07:58,5 (21) 16:31,5 (22) 25:12,3 02:43,8 97.17 5 45 3515356 Helena GUNTERN 2002 BSV / Sarsura Zernez 08:00,9 (24) 16:30,2 (21) 25:21,4 02:52,9 102.57 6 50 3515362 Bianca BUHOLZER 2002 ZSSV / Horw 08:00,3 (23) 16:37,7 (24) 25:24,4 02:55,9 104.35 7 46 3515342 Lola WUTHRICH -

Die Besten Tipps, Infos Und Ausflüge in Und Um Brig
Freizeitguide ... die besten Tipps, Infos und Ausflüge in und um Brig www.brig-simplon.ch · [email protected] · T: +41 (0) 27 921 60 30 Inhaltsverzeichnis Umkreis von 0-10 km S. 5 - 16 Umkreis von 11-20 km S. 17 - 20 Umkreis von 21-30 km S. 21 - 27 Umkreis von 31-40 km S. 28 - 33 Umkreis von 41-50 km S. 34 - 36 Umkreis ab 51 km S. 37 - 43 Legende und Erklärungen Um Ihnen die Orientierung zu Erleichtern haben wir die Ausflugstipps nach Distanzen und Himmelsrichtungen geordnet. Gemessen wurden die Entfernung jeweils ab Bahnhof Brig bis zu dem Punkt der per Auto am Reiseziel noch erreichbar ist. Auf folgende Symbole werden Sie in diesem Prospekt stossen: Anreisezeit bis zum genannten Ort mit dem Auto Distanz in Kilometer ab Bahnhof Brig mit dem Auto Um an den Ausflugstipp zu gelangen muss auf eine Berg- oder Zubringerbahn umgestiegen werden www.brig-simplon.ch Tel.: +41 (0)27 921 60 30 Änderungen bleiben vorbehalten. Für Druckfehler und Irrtümer, die bei der Herstellung des Prospekts unterlaufen sind, ist jede Haftung ausge- schlossen.Inseratekauf und Korrekturwünsche bitte per Mail an [email protected]. Stand September 2014 / BST AG Blatten 15 min Brig 8 min 15 min Visp Termen Ausflugstipps im Umkreis von 0 - 10 km ab Brig www.brig-simplon.ch · [email protected] · T: +41 (0) 27 921 60 30 5 Bauernmarkt Brig 0 min 0 km Jeden Samstagmorgen findet im Zentrum von Brig ein Bauernmarkt statt, an dem die Bioproduzenten aus der Region frisches Gemüse, Früchte, Fleischwaren und Milchprodukte verkaufen. -
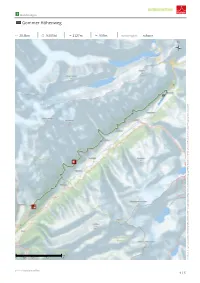
Gommer Höhenweg
Wanderungen Gommer Höhenweg 28,3km 9:20Std 1127m 935m Schwierigkeit schwer Kartengrundlagen: outdooractive Kartografie; OpenStreetMap: ©OpenStreetMap (www.openstreetmap.org) Mitwirkende, CC-BY-SA (www.creativecommons.org) ???copyright.swisstopo??? 1 / 5 Wanderungen Gommer Höhenweg Höhenprofil Asphalt 0,7km Schotterweg 2,3km Weg 3,9km Pfad 21,1km Unbekannt 0,1km Straße 0,2km Tourdaten Beste Jahreszeit Bewertungen Wanderung Schwierigkeit schwer Autoren Strecke 28,3 km Kondition Erlebnis Dauer 9:20 Std Technik Landschaft Community Aufstieg 1127 m Höhenlage Abstieg 935 m Weitere Tourdaten Verbindungen der Matterhorn Gotthard Bahn auch in einzelnen Teilabschnitten absolviert werden. Eigenschaften mit Bahn und Bus Der Gommer Höhenweg führt entlang der Sonnenseite aussichtsreich erreichbar des Hochtals Goms. Durch seine Lage einige Hundert Meter oberhalb der historischen Gommer Dörfer faunistische Highlights Streckentour garantiert die Wanderung Ausblicke über das gesamte botanische Highlights Bergbahnauf-/-abstieg Tal. Auszeichnungen (& Barrierefreiheit) In seiner ganzen Länge führt der Gommer Höhenweg Einkehrmöglichkeit über 28 Kilometer, er kann jedoch einfach in einzelne Teiletappen aufgeteilt werden. Die Bahnhöfe der Quelle Gommer Dörfer können mit einem jeweils 20 bis 30- Valais/Wallis Promotion minütigen Abstieg erreicht werden. Die historischen Rue Pré Fleuri 6 Dörfer mit den sonnenverbrannten Häusern sind auf 1951 Sion alle Fälle einen Besuch wert. Telefon +41 27 327 35 90 Fax +41 27 327 35 71 Der Weg führt hoch über dem Tal über blumenübersäte Weiden und durch Pinienwälder. Die Chancen, Wildtiere [email protected] zu beobachten, stehen hier besonders gut. Der Weg http://www.valais.ch führt ebenfalls durch einen Teil des Landschaftsparks Panoramareicher Höhenweg entlang der Sonnenseite Binntal. des oberen Rhonetals. Der Gommer Höhenweg besticht durch fantastische Aussichten auf die Ein Grossteil der Wälder auf der Route sind Walliser Gipfel. -

Urversammlung Infoblatt Der Gemeinde Goms
Infoblatt der Gemeinde Goms Ausgabe März 2019 und Infoschreiben zur ausserordentlichen Urversammlung vom 28. März 2019 Gluringen, 15. März 2019 Gemeinde Goms, Furkastrasse 35, 3998 Gluringen T +41 27 974 12 50, [email protected], www.gemeinde-goms.ch Die ausserordentliche Urversammlung wird einberufen auf: Datum: Donnerstag, 28. März 2019 Zeit: 19.00 Uhr Ort: Mehrzweckgebäude Gluringen Traktanden Urversammlung 1. Begrüssung 2. Wahl von zwei Stimmenzählern 3. Genehmigung Protokoll der letzten Urversammlung 4. Vorstellung und Genehmigung Kurtaxenreglement 5. Tagesschule Münster a. Abtretung Liegenschaften Primarschulhaus und Alte Turnhalle b. Genehmigung Statuten «Tagesschule Münster» 6. Information Reglemente 7. Verschiedenes Sämtliche Unterlagen zu den traktandierten Punkten liegen auf der Gemeinde Goms in Gluringen während den offiziellen Öffnungszeiten zur Einsichtnahme auf und können zudem auf unserer Homepage www.gemeinde-goms.ch eingesehen werden. Fahrgelegenheit: Bitte melden Sie sich bei der Gemeindekanzlei, falls Sie über keine Fahrmöglichkeit verfügen. Wir organisieren gerne den Transport. Gluringen, 1. März 2019 Gemeinde Goms Erläuterungen Traktanden der ausserordentlichen Urversammlung vom 28. März 2019 Traktandum 4: Vorstellen und Genehmigung Kurtaxenreglement Mit Urteil vom 8. Oktober 2018 hebt das Bundesgericht die Artikel in Bezug auf die ermittelte Durchschnittsbelegung von 57 Nächten der Kurtaxenreglemente der Fusionsge- meinden Goms und der Gemeinde Obergoms auf; die übrigen Beschwerdepunkte werden abgewiesen. In seinem Urteil definiert das Bundesgericht pro Gemeinde Richtwerte für die durchschnitt- liche Belegung und hält fest, dass «mit Blick auf die Dunkelziffer eine massvolle Aufrundung allenfalls noch haltbar sein dürfte». Die Gemeinden Goms und Obergoms haben, in Zusammenarbeit mit der Obergoms Tourismus AG und Vertretern der Hotellerie und der IG Zweitwohnungen, einen durch- schnittlichen Übernachtungssatz von 31 Nächten ermittelt. -

Langlaufen, Winterwandern Und Eisfeld
T +41 41 888 71 00 www.andermatt.ch www.andermatt.ch 00 00 71 71 888 888 41 41 +41 +41 T T Ferienregion Andermatt Andermatt Ferienregion Ferienregion Wichtige Hinweise • Verlassen Sie zum Schutz der Wildtiere und zu Ihrer eigenen Sicherheit keinesfalls die markierten Wege und Pfade. • Winterwanderern, Schneeschuhläufern und Hundehaltern ist es nicht erlaubt, die Langlaufloipen zu betreten. Finsteraahrhorn Lauteraahrhorn Dammastock Sustenhorn Bristen Oberalpstock Düssi Tödi Bifertenstock • Die Benutzung der Loipen, Winterwanderwege und Schnee- schuhtrails erfolgt auf eigene Gefahr. • Andermatt-Urserntal Tourismus GmbH lehnen jede Haftung ab. Galenstock Important notes Piz Giuv Basel Luzern Zürich Piz Ault • For the protection of wild animals and for your own safety do not leave the marked trails and paths. • Winter hikers, snowshoe walkers and dog owners are not Göschenen allowed to walk on the cross-country tracks. • The use of the cross-country tracks, the winter hiking trails and the snowshoe trails is at your own risk. Galmihorn Grimselpass Furkapass • Andermatt-Urserntal Tourismus GmbH disclaims any liability. Nätschen Oberalppass Note importanti Urserntal • Per la protezione degli animali selvatici e per la loro sicurezza Realp Sedrun non lasciare i sentieri segnalati. Andermatt Rueras • Sulle piste di fondo è proibito camminare a piedi o con le Hospental Dieni Gemsstock Bugnei Disentis/ Mustér ciaspole, così come portare i cani. Tschamutt-Selva Disla Oberwald Acla da • L‘utilizzo delle piste di fondo,dei sentieri invernali e delle Furka-Basistunnel Segnas Fontauna Sumvitg- ski de fond, randonnées d‘hiver et champ de glace de champ et d‘hiver randonnées fond, de ski Obergesteln Pizzo Centrale Cumpadials Campliun passeggiate con le ciaspole, avviene a proprio rischio e Mumpé- Pardomat Rabius Ulrichen Sedrun Cavadiras pericolo. -

Regionalezust Ä Ndigkeit 2 0
Departement für Volkswirtschaft und Bildung Öffnungszeiten Sekretariat: Kantonale Dienststelle für die Jugend Montag – Freitag: 09.00 – 12.00 Uhr und 14.00 – 17.00 Uhr Zentrum für Entwicklung und Therapie des Brig 027 606 99 30 Kindes und Jugendlichen (ZET) E-Mail: [email protected] ZET REGION BRIG : REGIONALE ZUSTÄNDI G K E I T 2020 – 2021 Anstellungsprozente und Funktion Mitarbeiterin Zuständig für die Region/en Zuständig für die Gemeinden Arbeitstage 100%: Montag, Dienstag, Leiterin ZET Brig Süd Brig, Brig-Glis Therese ZENHÄUSERN Mittwoch, Donnerstag, Psychologin St. Niklaus Grächen, Herbriggen, St. Niklaus Freitag Morgen Brig Süd Brig-Glis, Brigerbad, Gamsen, Glis 50%: Dienstag, Mittwoch, Psychologin Bettina BUMANN Mörel-Aletsch Betten, Bettmeralp, Bister, Bitsch, Grengiols, Mörel-Filet, Ried-Mörel Donnerstag Morgen Brig Süd Brig, Brig-Glis, Ried-Brig, Termen, Simplon-Dorf, Zwischbergen 85%: Montag, Dienstag, Mittwoch, Psychologin Diana AUGUSTO COELHO Zermatt Randa, Täsch, Zermatt Donnerstag Kinder- und Jugendeinrichtung Mattini Kinder- und Jugendeinrichtung Mattini Psychologin Svjetlana ABGOTTSPON Naters Birgisch, Mund, Naters 20%: Montag Naters Naters Obergoms-Münster Blitzingen, Grafschaft, Münster-Geschinen, Niederwald, Obergoms, Psychologin Maria-Gabriella WERLEN 60%: Dienstag, Mittwoch, Freitag Reckingen-Gluringen Unnergoms-Fiesch Bellwald, Binn, Ernen, Fiesch, Fieschertal, Lax Psychologin Maria Laura ZENGAFFINEN Brig Süd Brig, Brig-Glis, Glis 40%: Mittwoch, Donnerstag Logopädin Chantal SCHNYDER Zermatt Randa, Täsch -

Facts & Figures Andermatt Holiday Region
Facts & Figures Andermatt Holiday Region Content Welcome..................................................................................................................... 1 1. The six municipalities ........................................................................................... 3 Andermatt .............................................................................................................. 3 Hospental ............................................................................................................... 3 Realp ..................................................................................................................... 3 Göschenen ............................................................................................................. 4 Wassen .................................................................................................................. 4 Gurtnellen .............................................................................................................. 4 2. Getting here ......................................................................................................... 5 3. Facts and Figures ................................................................................................. 7 Accommodation and Restaurants ............................................................................... 8 Key figures - Hotels ................................................................................................ 10 Overnight stays 2019 ............................................................................................. -

Big Data for Analyses of Small-Scale Regional Variation: a Case Study on Sound Change in Swiss German
Big Data for analyses of small-scale regional variation: A case study on sound change in Swiss German Adrian Leemann1, Marie-José Kolly2 1Phonetics Lab., Department of Theoretical and Applied Linguistics, University of Cambridge 2Phonetics Lab., Department of Comparative Linguistics, University of Zurich [email protected], [email protected] Abstract 1.1. Previous studies In this case study we examine sound change of Altoberdeutsch Only a few studies have examined how SwG dialects have <iu> in Swiss German dialects. We used contemporary dialect changed since the Atlas. [5] and [6] reported change in the data from nearly 60,000 speakers – collected with the lexicon. The latter found convergence tendencies towards smartphone app Dialäkt Äpp – and compared it to historical Standard German and showed that younger speakers deviated Atlas data from the 1950s. Results revealed hierarchical and from the Atlas more than older speakers in lexical features. contra-hierarchical diffusion patterns for some dialectal Similarly, [5] conducted an online survey with 9000 variants, while other variants remained virtually unchanged participants. Based on this study, [7] as well as [8] presented over the course of seven decades. We further report change in dialect maps that corroborate tendencies of leveling in the apparent time, with older speakers using traditional variants lexicon for some of the variables examined. For more frequently than younger speakers. Using this case study morphosyntax, [9] found that only little change has occurred as a model, future work using the Dialäkt Äpp corpus will for the constructions examined. A number of studies have reveal patterns of feature diffusion and dialect leveling on a further reported sound change over the past decades, such as larger scale. -

ZETR Egion B Rig : R Egionale Z Ust Ä Ndigkeit 2 0
Departement für Volkswirtschaft und Bildung Öffnungszeiten Sekretariat: Kantonale Dienststelle für die Jugend Montag – Freitag: 08.00 – 12.00 Uhr und 13.30 – 17.00 Uhr Zentrum für Entwicklung und Therapie des Brig 027 606 99 30 Fax 027 606 99 34 Kindes und Jugendlichen (ZET) E-Mail: [email protected] ZET Region Brig: Regionale Zuständigkeit 2018 – 2 0 1 9 Anstellungsprozente und Funktion Mitarbeiterin Zuständig für die Region/en Zuständig für die Gemeinden Arbeitstage Leiterin ZET Brig Brig Süd Brig, Brig-Glis, Termen, Simplon, Zwischbergen Therese Zenhäusern 100%: Montag bis Freitag Mittag Psychologin St. Niklaus Grächen, Herbriggen, St. Niklaus Brig Süd Brig-Glis, Brigerbad, Gamsen, Glis, 50% : Dienstag, Mittwoch, Psychologin Bettina Bumann Mörel-Aletsch Betten, Bettmeralp, Bister, Bitsch, Grengiols, Mörel-Filet, Ried-Mörel Donnerstag Morgen Brig Süd Ried-Brig 55%: Dienstag, Mittwoch Psychologin Diana Augusto-Coelho Zermatt Randa, Täsch, Zermatt Nachmittag, Donnerstag Blitzingen, Grafschaft, Münster-Geschinen, Niederwald, Obergoms, Goms, Obergoms Reckingen-Gluringen Psychologin Gabriella Werlen Fiesch 60%: Dienstag, Mittwoch, Freitag Bellwald, Binn, Ernen, Fiesch, Fieschertal, Lax Naters Birgisch, Mund, Naters Brig Süd Brig-Glis 70%: Montag, Dienstag Morgen, Logopädin Anya Lindemann St. Niklaus Grächen, Herbriggen, St. Niklaus Mittwoch, Donnerstag Zermatt Täsch, Randa Brig-Glis 50%: Montag, Dienstag, Logopädin Chantal Oggier Brig Süd Ried-Brig, Simplon-Dorf, Termen, Zwischbergen Freitag Morgen 55%: Montag, Mittwoch Morgen, Logopädin -

Gourmet Bike
Gourmet - Bike 4 GENUSS PUR 3 2 KULINARISCHES 1 FAHRRADFAHREN Fahren Sie entspannt durch die malerische Landschaft und kehren Sie dabei in ausgewählte Gastronomiebetriebe des Goms ein. Belohnen Sie Ihren Gaumen nach jeder Etappe und verwöhnen ihn mit kulinarischen Köstlichkeiten aus Obergesteln der Region, ganz nach der Philosophie des Hotelleriekönigs OBERGOMS TOURISMUS AG Cäsar Ritz aus Niederwald. 1. Hotel-Restaurant Hubertus Furkastrasse 53 7 1 CH-3985 Münster Gültigkeit: Anfang Juni bis Mitte Oktober 6 5 Geschinen +41 27 974 68 68 Distanz: 30 km [email protected] Reine Fahrzeit: ca. 2-3 Stunden 2. Restaurant Baschi www.obergoms.ch Münster 3. Hotel-Restaurant Landhaus 4. Hotel-Restaurant Croix d‘Or et Poste Gluringen 5. Hotel-Restaurant Tenne Blitzingen 6. Hotel-Restaurant Castle Niederwald 8 7. Hotel-Restaurant Drei Tannen 9 Ernen 8. Restaurant Erner Garten 9. Restaurant Gommerstuba Ihr Gommer Gourmet - Bike Menü VORSPEISE (11.15 - 12.15 UHR) Hotel - Restaurant Hubertus, Obergesteln Hotel - Restaurant Castle, Blitzingen (auf Anmeldung, +41 27 970 17 00) Gault Millau (15 Punkte) Cäsar Ritz-Betrieb und Gault Millau (16 Punkte) Täglich geöffnet Ruhetag: Montag nur im Oktober Schmorbraten-Siedfleisch-Terrine mit Meerrettich und Salat Lammrücken mit Kräuterkruste auf Gemüse mit Risotto oder Himmel und Erde mit Entenleber und Salat DESSERT (13.30 - 17.00 UHR) Restaurant Baschi, Geschinen Gault Millau (12 Punkte) Hotel - Restaurant Drei Tannen, Niederwald Ruhetag: Sonntag und Montag Cäsar Ritz-Betrieb Ruhetag: Mittwoch, von Mitte Juli