'All Science Is Either Physics Or Stamp Collecting' This Is a Quote Attributed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Department of Physics College of Arts and Sciences Physics
DEPARTMENT OF PHYSICS COLLEGE OF ARTS AND SCIENCES PHYSICS Faculty I. Major in Physics—38 hours William Nettles (2006). Professor of Physics, Department A. Physics 231-232, 311, 313, 314, 420, 424(1-3 Chair, and Associate Dean of the College of Arts and hours), 430, 498—28–30 hours Sciences. B.S., Mississippi College; M.S., and Ph.D., B. Select three or more courses: PHY 262, 325, 350, Vanderbilt University. 360, 395-6-7*, 400, 410, 417, 425 (1-2 hours**), 495* Ildefonso Guilaran (2008). Associate Professor of Physics. C. Prerequisites: MAT 211, 212, 213, 314 B.S., Western Kentucky University; M.S. and Ph.D., *Must be approved Special/Independent Studies Florida State University. **Maximum 3 hours from 424 and 425 apply to major. Geoffrey Poore (2010). Assistant Professor of Physics. B.A., II. Major in Physical Science—44 hours Wheaton College; M.S. and Ph.D., University of Illinois. A. CHE 111, 112, 113, 211, 221—15 hours David A. Ward (1992, 1999). Professor of Physics, B.S. B. PHY 112, 231-32, 311, 310 or 301—22 hours and M.A., University of South Florida; Ph.D., North C. Upper Level Electives from CHE and PHY—7 Carolina State University. hours; maximum 1 hour from 424 and 1 from 498 III. Minor in Physics—24 semester hours Staff Physics 231-232, 311, + 10 hours of Physics electives Christine Rowland (2006). Academic Secretary— except PHY 111, 112, 301, 310 Engineering, Physics, Math, and Computer Science. IV. Teacher Licensure in Physics (Grades 6–12) A. Complete the requirements shown above for the Physics or Physical Science major. -

Fundamentals of Particle Physics
Fundamentals of Par0cle Physics Particle Physics Masterclass Emmanuel Olaiya 1 The Universe u The universe is 15 billion years old u Around 150 billion galaxies (150,000,000,000) u Each galaxy has around 300 billion stars (300,000,000,000) u 150 billion x 300 billion stars (that is a lot of stars!) u That is a huge amount of material u That is an unimaginable amount of particles u How do we even begin to understand all of matter? 2 How many elementary particles does it take to describe the matter around us? 3 We can describe the material around us using just 3 particles . 3 Matter Particles +2/3 U Point like elementary particles that protons and neutrons are made from. Quarks Hence we can construct all nuclei using these two particles -1/3 d -1 Electrons orbit the nuclei and are help to e form molecules. These are also point like elementary particles Leptons We can build the world around us with these 3 particles. But how do they interact. To understand their interactions we have to introduce forces! Force carriers g1 g2 g3 g4 g5 g6 g7 g8 The gluon, of which there are 8 is the force carrier for nuclear forces Consider 2 forces: nuclear forces, and electromagnetism The photon, ie light is the force carrier when experiencing forces such and electricity and magnetism γ SOME FAMILAR THE ATOM PARTICLES ≈10-10m electron (-) 0.511 MeV A Fundamental (“pointlike”) Particle THE NUCLEUS proton (+) 938.3 MeV neutron (0) 939.6 MeV E=mc2. Einstein’s equation tells us mass and energy are equivalent Wave/Particle Duality (Quantum Mechanics) Einstein E -

PHOTONICS and PLASMONICS SEMINAR – SPRING 2011 Unit Value: 1 Instructor: Prof
EE298‐5 PHOTONICS AND PLASMONICS SEMINAR – SPRING 2011 unit value: 1 instructor: Prof. Ivan Kaminow, [email protected] coordinator: Lea Barker, [email protected] class time: FRIDAYS, 11:00AM ‐ 12:30, 521 CORY HALL pre‐requisite: An interest in Photonics and/or Plasmonics. May be taken for credit and/or fun. website: http://inst.eecs.berkeley.edu/~ee298‐5/sp11/ plasmonics list: [email protected] This course is intended to give students at the advanced undergraduate or graduate level, and researchers, insight into current research based on a series of invited talks. SCHEDULE: 1/21 ‐ Prof. REUVEN GORDON, U. Victoria, Canada, “Challenging the Limits of Diffraction” Abstract: This talk will show ways of challenging three limits of optical diffraction. First, it will be shown how to focus light below the Abbe diffraction limit [1]. Next it will be shown how to squeeze light through small holes in a metal screen, allowing for 100% transmission in some cases, in contrast to Betheʹs aperture theory as found in textbooks [2,3]. This has interesting applications for biosensors. Finally, it will be shown how to optically trap nanoparticles with powers orders of magnitude smaller than required by conventional by Rayleigh scattering formulations [4], which has interesting applications for manipulating viruses and quantum dots. Theoretical and experimental results will be presented. (1) R. Gordon, ʺProposal for superfocusing at visible wavelengths using radiationless interference of a plasmonic array,ʺ Physical Review Letters, 102, 207402 (2009). (2) R. Gordon, A. G. Brolo, A. McKinnon, A. Rajora, B. Leathem, and K. L. Kavanagh, ʺStrong polarization in the optical transmission through elliptical nanohole arraysʺ Physical Review Letters, 92, 037401, (2004). -

A Comprehensive Framework to Reinforce Evidence Synthesis Features in Cloud-Based Systematic Review Tools
applied sciences Article A Comprehensive Framework to Reinforce Evidence Synthesis Features in Cloud-Based Systematic Review Tools Tatiana Person 1,* , Iván Ruiz-Rube 1 , José Miguel Mota 1 , Manuel Jesús Cobo 1 , Alexey Tselykh 2 and Juan Manuel Dodero 1 1 Department of Informatics Engineering, University of Cadiz, 11519 Puerto Real, Spain; [email protected] (I.R.-R.); [email protected] (J.M.M.); [email protected] (M.J.C.); [email protected] (J.M.D.) 2 Department of Information and Analytical Security Systems, Institute of Computer Technologies and Information Security, Southern Federal University, 347922 Taganrog, Russia; [email protected] * Correspondence: [email protected] Abstract: Systematic reviews are powerful methods used to determine the state-of-the-art in a given field from existing studies and literature. They are critical but time-consuming in research and decision making for various disciplines. When conducting a review, a large volume of data is usually generated from relevant studies. Computer-based tools are often used to manage such data and to support the systematic review process. This paper describes a comprehensive analysis to gather the required features of a systematic review tool, in order to support the complete evidence synthesis process. We propose a framework, elaborated by consulting experts in different knowledge areas, to evaluate significant features and thus reinforce existing tool capabilities. The framework will be used to enhance the currently available functionality of CloudSERA, a cloud-based systematic review Citation: Person, T.; Ruiz-Rube, I.; Mota, J.M.; Cobo, M.J.; Tselykh, A.; tool focused on Computer Science, to implement evidence-based systematic review processes in Dodero, J.M. -

Molecular Biology for Computer Scientists
CHAPTER 1 Molecular Biology for Computer Scientists Lawrence Hunter “Computers are to biology what mathematics is to physics.” — Harold Morowitz One of the major challenges for computer scientists who wish to work in the domain of molecular biology is becoming conversant with the daunting intri- cacies of existing biological knowledge and its extensive technical vocabu- lary. Questions about the origin, function, and structure of living systems have been pursued by nearly all cultures throughout history, and the work of the last two generations has been particularly fruitful. The knowledge of liv- ing systems resulting from this research is far too detailed and complex for any one human to comprehend. An entire scientific career can be based in the study of a single biomolecule. Nevertheless, in the following pages, I attempt to provide enough background for a computer scientist to understand much of the biology discussed in this book. This chapter provides the briefest of overviews; I can only begin to convey the depth, variety, complexity and stunning beauty of the universe of living things. Much of what follows is not about molecular biology per se. In order to 2ARTIFICIAL INTELLIGENCE & MOLECULAR BIOLOGY explain what the molecules are doing, it is often necessary to use concepts involving, for example, cells, embryological development, or evolution. Bi- ology is frustratingly holistic. Events at one level can effect and be affected by events at very different levels of scale or time. Digesting a survey of the basic background material is a prerequisite for understanding the significance of the molecular biology that is described elsewhere in the book. -

Engineering Physics I Syllabus COURSE IDENTIFICATION Course
Engineering Physics I Syllabus COURSE IDENTIFICATION Course Prefix/Number PHYS 104 Course Title Engineering Physics I Division Applied Science Division Program Physics Credit Hours 4 credit hours Revision Date Fall 2010 Assessment Goal per Outcome(s) 70% INSTRUCTION CLASSIFICATION Academic COURSE DESCRIPTION This course is the first semester of a calculus-based physics course primarily intended for engineering and science majors. Course work includes studying forces and motion, and the properties of matter and heat. Topics will include motion in one, two, and three dimensions, mechanical equilibrium, momentum, energy, rotational motion and dynamics, periodic motion, and conservation laws. The laboratory (taken concurrently) presents exercises that are designed to reinforce the concepts presented and discussed during the lectures. PREREQUISITES AND/OR CO-RECQUISITES MATH 150 Analytic Geometry and Calculus I The engineering student should also be proficient in algebra and trigonometry. Concurrent with Phys. 140 Engineering Physics I Laboratory COURSE TEXT *The official list of textbooks and materials for this course are found on Inside NC. • COLLEGE PHYSICS, 2nd Ed. By Giambattista, Richardson, and Richardson, McGraw-Hill, 2007. • Additionally, the student must have a scientific calculator with trigonometric functions. COURSE OUTCOMES • To understand and be able to apply the principles of classical Newtonian mechanics. • To effectively communicate classical mechanics concepts and solutions to problems, both in written English and through mathematics. • To be able to apply critical thinking and problem solving skills in the application of classical mechanics. To demonstrate successfully accomplishing the course outcomes, the student should be able to: 1) Demonstrate knowledge of physical concepts by their application in problem solving. -

Outline of Science
Outline of science The following outline is provided as a topical overview of • Empirical method – science: • Experimental method – The steps involved in order Science – systematic effort of acquiring knowledge— to produce a reliable and logical conclusion include: through observation and experimentation coupled with logic and reasoning to find out what can be proved or 1. Asking a question about a natural phenomenon not proved—and the knowledge thus acquired. The word 2. Making observations of the phenomenon “science” comes from the Latin word “scientia” mean- 3. Forming a hypothesis – proposed explanation ing knowledge. A practitioner of science is called a for a phenomenon. For a hypothesis to be a "scientist". Modern science respects objective logical rea- scientific hypothesis, the scientific method re- soning, and follows a set of core procedures or rules in or- quires that one can test it. Scientists generally der to determine the nature and underlying natural laws of base scientific hypotheses on previous obser- the universe and everything in it. Some scientists do not vations that cannot satisfactorily be explained know of the rules themselves, but follow them through with the available scientific theories. research policies. These procedures are known as the 4. Predicting a logical consequence of the hy- scientific method. pothesis 5. Testing the hypothesis through an experiment – methodical procedure carried out with the 1 Essence of science goal of verifying, falsifying, or establishing the validity of a hypothesis. The 3 types of -

Principal Scientist, Pharmacology (DMPK) at Entrada Therapeutics Full-Time Opportunity in Boston, MA
Principal Scientist, Pharmacology (DMPK) at Entrada Therapeutics Full-Time Opportunity in Boston, MA Company Overview Entrada Therapeutics is a biotechnology company dedicated to transforming the treatment of devastating diseases through the intracellular delivery of biologics. Entrada’s technology enables the efficient intracellular delivery of proteins, peptides and nucleic acids, thus allowing for the development of programs across several intracellular target classes. The Company’s novel approach addresses current challenges associated with both large and small molecule therapeutics and represents a fundamental advancement in the delivery of molecules into the cytosol. Entrada is comprised of experts and leaders in both biologics development and the rare disease space. In December 2018, Entrada closed a $59 million Series A from 5AM Ventures, MPM Capital, Roche Venture Fund, MRL Ventures Fund and Agent Capital. Position Summary Entrada is hiring for a highly motivated, collaborative and innovative Principal Scientist, specializing in pharmacokinetics and pharmacology to join the Company’s intracellular biologics discovery and development efforts. The candidate will work with a world-class, multidisciplinary team of drug discovery scientists and lead the in vitro and in vivo ADME, PK, PD and toxicokinetic efforts. Specifically, the candidate will design, manage and execute in vitro and in vivo programs both in- house and at contract research organizations. The candidate will also be responsible for analyzing, interpreting and modeling -

How Science Works
PB 1 How science works The Scientific Method is traditionally presented in the first chapter of science text- books as a simple recipe for performing scientific investigations. Though many use- ful points are embodied in this method, it can easily be misinterpreted as linear and “cookbook”: pull a problem off the shelf, throw in an observation, mix in a few ques- tions, sprinkle on a hypothesis, put the whole mixture into a 350° experiment—and voila, 50 minutes later you’ll be pulling a conclusion out of the oven! That might work if science were like Hamburger Helper®, but science is complex and cannot be re- duced to a single, prepackaged recipe. The linear, stepwise representation of the process of science is simplified, but it does get at least one thing right. It captures the core logic of science: testing ideas with evidence. However, this version of the scientific method is so simplified and rigid that it fails to accurately portray how real science works. It more accurately describes how science is summarized after the fact—in textbooks and journal articles—than how sci- ence is actually done. The simplified, linear scientific method implies that scientific studies follow an unvarying, linear recipe. But in reality, in their work, scientists engage in many different activities in many different sequences. Scientific investigations often involve repeating the same steps many times to account for new information and ideas. The simplified, linear scientific method implies that science is done by individual scientists working through these steps in isolation. But in reality, science depends on interactions within the scientific community. -
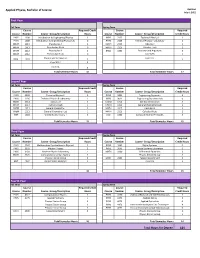
Physics, Applied Physics, B.S
Applied Physics, Bachelor of Science Updated July 6, 2021 First Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description Credit Hours PHYS 1308 Introduction to Engineering/Physics 3 PHYS 2325 Technical Physics I 3 PHYS 1008 Introduction to Engineering/Physics Lab 0 PHYS 2125 Technical Physics I Laboratory 1 MATH 2211 Precalculus A 2 MATH 2313 Calculus I 3 MATH 2011 Precalculus A Lab 0 MATH 2113 Calculus I Lab 1 MATH 2212 Precalculus B 2 ENGL 1302 Research and Argument 3 MATH 2012 Precalculus B Lab 0 Core S/BS 3 ENGL 1301 Rhetoric and Composition 3 Core LPC 3 Core SPCH 3 Core CA 3 Total Semester Hours 16 Total Semester Hours 17 Second Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description Credit Hours PHYS 2326 Technical Physics II 3 PHYS 3421 Engineering Dynamics 4 PHYS 2126 Technical Physics II Laboratory 1 PHYS 3021 Engineering Dynamics Lab 0 MATH 2314 Calculus II 3 CHEM 1312 General Chemistry II 3 MATH 2114 Calculus II Lab 1 CHEM 1112 General Chemistry II Lab 1 CHEM 1311 General Chemistry I 3 MATH 3315 Calculus III 3 CHEM 1111 General Chemistry I Lab 1 MATH 3115 Calculus III Lab 1 HIST 1301 United States History I 3 CSCI 1302 Computer Science Principles 3 Total Semester Hours 15 Total Semester Hours 15 Third Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description -

Marko Lončar
MARKO LONČAR Harvard University http://nano-optics.seas.harvard.edu School of Engineering And Applied Sciences [email protected] Pierce Hall, 107C (617) 495-5798 29 Oxford Street Cambridge, MA 02138 Career History Harvard College Professor May 3, 2017 - present Harvard University Tiantsai Lin Professor of Electrical Engineering Jul. 1st, 2012 – present Harvard University Associate Professor of Electrical Engineering Jul. 1st, 2010 – Jun. 30th, 2012 Harvard University Assistant Professor of Electrical Engineering Jul. 1st, 2006 – Jun. 30th, 2010 Harvard University Postdoctoral Scholar in Applied Physics Oct. 1st, 2003 – Jun. 31st, 2006 Harvard University; Adviser: Federico Capasso Education Ph.D. in Electrical Engineering, California Institute of Technology 1998-2003 Thesis “Nanophotonics devices based on planar photonic crystals”. Adviser: Axel Scherer M.S. in Electrical Engineering, California Institute of Technology 1997-1998 Power electronics group. Adviser: Slobodan Ćuk Diploma in Electrical Engineering, University of Belgrade (R. Serbia) 1992-1997 Alfred P. Sloan Research Fellowship 02/16/2010 Awards and Recognitions NSF CAREER Award 2/1/2009 – 1/31/2014 Kavli Fellow (2013 US Kavli Frontiers of Science participant) November 2013 Levenson Prize for Excellence in Undergraduate Teaching, Harvard University 2011/12 academic year Fellow of Optical Society of America, Senior Member of IEEE and SPIE Research We study and engineer light-matter interaction in nanostructures, and apply these effects to develop novel and/or Interests better performing devices & systems. Examples include quantum photonics with diamond color centers, nonlinear nanophotonics (e.g. frequency combs, quantum wavelength conversion), behavior of light in composite metamaterials designed via topology optimization (inverse design), etc. Our efforts span a variety of optical materials (e.g.