Materials Science and Engineering 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PHOTONICS and PLASMONICS SEMINAR – SPRING 2011 Unit Value: 1 Instructor: Prof
EE298‐5 PHOTONICS AND PLASMONICS SEMINAR – SPRING 2011 unit value: 1 instructor: Prof. Ivan Kaminow, [email protected] coordinator: Lea Barker, [email protected] class time: FRIDAYS, 11:00AM ‐ 12:30, 521 CORY HALL pre‐requisite: An interest in Photonics and/or Plasmonics. May be taken for credit and/or fun. website: http://inst.eecs.berkeley.edu/~ee298‐5/sp11/ plasmonics list: [email protected] This course is intended to give students at the advanced undergraduate or graduate level, and researchers, insight into current research based on a series of invited talks. SCHEDULE: 1/21 ‐ Prof. REUVEN GORDON, U. Victoria, Canada, “Challenging the Limits of Diffraction” Abstract: This talk will show ways of challenging three limits of optical diffraction. First, it will be shown how to focus light below the Abbe diffraction limit [1]. Next it will be shown how to squeeze light through small holes in a metal screen, allowing for 100% transmission in some cases, in contrast to Betheʹs aperture theory as found in textbooks [2,3]. This has interesting applications for biosensors. Finally, it will be shown how to optically trap nanoparticles with powers orders of magnitude smaller than required by conventional by Rayleigh scattering formulations [4], which has interesting applications for manipulating viruses and quantum dots. Theoretical and experimental results will be presented. (1) R. Gordon, ʺProposal for superfocusing at visible wavelengths using radiationless interference of a plasmonic array,ʺ Physical Review Letters, 102, 207402 (2009). (2) R. Gordon, A. G. Brolo, A. McKinnon, A. Rajora, B. Leathem, and K. L. Kavanagh, ʺStrong polarization in the optical transmission through elliptical nanohole arraysʺ Physical Review Letters, 92, 037401, (2004). -

Implementing NIR for Polymer Production Process Monitoring Dr
Implementing NIR for Polymer Production Process Monitoring Dr. Hari Narayanan, Senior Product Manager – Spectroscopy, Metrohm USA Inc Introduction while at the bench or pilot plant level it can be used to Polymers are a very important class of organic materials, optimize reaction conditions. Finally, when used in a widely used in many fields due to their unique properties complete feedback-control-loop at the industrial reactor such as tensile strength and viscoelastic behavior under level, significant improvement in product quality can be deformation. With more stringent demands on the quality expected, as well as savings of non-renewable resources, of polymer products and pressure to reduce cost in energy, reactor and personnel time. Creating an effective production and processing, the need for fast, accurate and NIR implementation strategy depends on the type of reliable monitoring methods is greater than ever. polymerization process, the properties of interest, and measurement conditions. Analytical methods, which require time-consuming and intensive sample preparation, are difficult to implement Polymerization Processes effectively in a process or quality control environment, NIR spectroscopy can be used for bulk, solution, emulsion and waste precious time and materials. Efficient process and suspension polymerization monitoring, providing control demands a measurement technique which is information on copolymer composition and distribution, rapid, requires little or no sample preparation and minimal monomer conversion, molecular weight averages, average technical expertise. It should be reliable, rugged and easily particle sizes and more. automated. Bulk polymerization Near-infrared (NIR) spectroscopy meets those needs by In bulk polymerization, the reaction is carried out in the being both rapid and precise. -
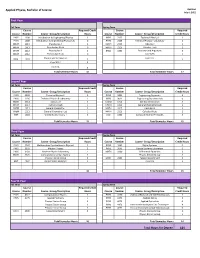
Physics, Applied Physics, B.S
Applied Physics, Bachelor of Science Updated July 6, 2021 First Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description Credit Hours PHYS 1308 Introduction to Engineering/Physics 3 PHYS 2325 Technical Physics I 3 PHYS 1008 Introduction to Engineering/Physics Lab 0 PHYS 2125 Technical Physics I Laboratory 1 MATH 2211 Precalculus A 2 MATH 2313 Calculus I 3 MATH 2011 Precalculus A Lab 0 MATH 2113 Calculus I Lab 1 MATH 2212 Precalculus B 2 ENGL 1302 Research and Argument 3 MATH 2012 Precalculus B Lab 0 Core S/BS 3 ENGL 1301 Rhetoric and Composition 3 Core LPC 3 Core SPCH 3 Core CA 3 Total Semester Hours 16 Total Semester Hours 17 Second Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description Credit Hours PHYS 2326 Technical Physics II 3 PHYS 3421 Engineering Dynamics 4 PHYS 2126 Technical Physics II Laboratory 1 PHYS 3021 Engineering Dynamics Lab 0 MATH 2314 Calculus II 3 CHEM 1312 General Chemistry II 3 MATH 2114 Calculus II Lab 1 CHEM 1112 General Chemistry II Lab 1 CHEM 1311 General Chemistry I 3 MATH 3315 Calculus III 3 CHEM 1111 General Chemistry I Lab 1 MATH 3115 Calculus III Lab 1 HIST 1301 United States History I 3 CSCI 1302 Computer Science Principles 3 Total Semester Hours 15 Total Semester Hours 15 Third Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description -

Marko Lončar
MARKO LONČAR Harvard University http://nano-optics.seas.harvard.edu School of Engineering And Applied Sciences [email protected] Pierce Hall, 107C (617) 495-5798 29 Oxford Street Cambridge, MA 02138 Career History Harvard College Professor May 3, 2017 - present Harvard University Tiantsai Lin Professor of Electrical Engineering Jul. 1st, 2012 – present Harvard University Associate Professor of Electrical Engineering Jul. 1st, 2010 – Jun. 30th, 2012 Harvard University Assistant Professor of Electrical Engineering Jul. 1st, 2006 – Jun. 30th, 2010 Harvard University Postdoctoral Scholar in Applied Physics Oct. 1st, 2003 – Jun. 31st, 2006 Harvard University; Adviser: Federico Capasso Education Ph.D. in Electrical Engineering, California Institute of Technology 1998-2003 Thesis “Nanophotonics devices based on planar photonic crystals”. Adviser: Axel Scherer M.S. in Electrical Engineering, California Institute of Technology 1997-1998 Power electronics group. Adviser: Slobodan Ćuk Diploma in Electrical Engineering, University of Belgrade (R. Serbia) 1992-1997 Alfred P. Sloan Research Fellowship 02/16/2010 Awards and Recognitions NSF CAREER Award 2/1/2009 – 1/31/2014 Kavli Fellow (2013 US Kavli Frontiers of Science participant) November 2013 Levenson Prize for Excellence in Undergraduate Teaching, Harvard University 2011/12 academic year Fellow of Optical Society of America, Senior Member of IEEE and SPIE Research We study and engineer light-matter interaction in nanostructures, and apply these effects to develop novel and/or Interests better performing devices & systems. Examples include quantum photonics with diamond color centers, nonlinear nanophotonics (e.g. frequency combs, quantum wavelength conversion), behavior of light in composite metamaterials designed via topology optimization (inverse design), etc. Our efforts span a variety of optical materials (e.g. -

MSE 403: Ceramic Materials
MSE 403: Ceramic Materials Course description: Processing, characteristics, microstructure and properties of ceramic materials. Number of credits: 3 Course Coordinator: John McCloy Prerequisites by course: MSE 201 Prerequisites by topic: 1. Basic knowledge of thermodynamics. 2. Elementary crystallography and crystal structure. 3. Mechanical behavior of materials. Postrequisites: None Textbooks/other required 1. Carter, C.B. and Norton, M.G. Ceramic Materials Science and Engineering, materials: Springer, 2007. Course objectives: 1. Review of crystallography and crystal structure. 2. Review of structure of atoms, molecules and bonding in ceramics. 3. Discussion on structure of ceramics. 4. Effects of structure on physical properties. 5. Ceramic Phase diagrams. 6. Discussion on defects in ceramics. 7. Introduction to glass. 8. Discussion on processing of ceramics. 9. Introduction to sintering and grain growth. 10. Introduction to mechanical properties of ceramics. 11. Introduction to electrical properties of ceramics. 12. Introduction to bioceramics. 13. Introduction to magnetic ceramics. Topics covered: 1. Introduction to crystal structure and crystallography. 2. Fundamentals of structure of atoms. 3. Structure of ceramics and its influence on properties. 4. Binary and ternary phase diagrams. 5. Point defects in ceramics. 6. Glass and glass-ceramic composites. 7. Ceramics processing and sintering. 8. Mechanical properties of ceramics. 9. Electrical properties of ceramics. 10. Bio-ceramics. 11. Ceramic magnets. Expected student outcomes: 1. Knowledge of crystal structure of ceramics. 2. Knowledge of structure-property relationship in ceramics. 3. Knowledge of the defects in ceramics (Point defects). 4. Knowledge of glass and glass-ceramic composite materials. 5. Introductory knowledge on the processing of bulk ceramics. 6. Applications of ceramic materials in structural, biological and electrical components. -

Rheology Bulletin
RHEOLOGY BULLETIN izoLvza §ei Publication of the SOCIETY OF RHEOLOGY Vol. XII No. 1 February 1941 « RHEOLOGY BULLETIN Published Quarterly by THE SOCIETY OF RHEOLOGY Dedicated to the Development of the Science of the Deformation and Flow of Matter 175 Fifth Avenue New York, N. Y. THE SOCIETY OF RHEOLOGY IS ONE OF THE FIVE FOUNDER SOCIETIES OF THE AMERICAN INSTITUTE OF PHYSICS A. STUART HUNTER, President WHEELER P. DAVEY, Editor Rayon Department Room 6, New Physics Pldg. E. I. du Pont de Nemours & Company The Pennsylvania State College Buffalo, New York State College, Pennsylvania J. H. DILLON, First Vice-President H. F. WAKEFIELD, Publishing Editor Firestone Tire & Rubber Company Bakelite Corporation Akron, Ohio Bloomfield, New Jersey R. H. EWELL, Second Vice-President H. R. LILLIE, Secretary-Treasurer Purdue University Corning Glass Works Lafayette, Indiana Corning, New York Non-member Subscriptions: $2.00 Annually Single Copies: 75c Apiece Adress All Communications to the Editor RHEOLOGY BULLETIN Vol. XII No. 1 TTdvra gel February 1941 TABLE OF CONTENTS Page Rheological News - 2 Report on Conference on "Viscosity" H. Mark, Polytechnic Institute of Brooklyn 3 Report of Committe on Definitions 6 Correlated Abstracts —« 12 Viscosity Flow of Metals Geological Applications Theory Plastics Contributed Papers The Viscosity of Helium II A. D. Misener, University of Toronto — 1 > The Transient Resilience of Some Fluids J. M. Kendall, Geophysical Corp 2( 1. The Leaflet Becomes The Bulletin. Commencing with this issue, the Rheology Leaflet becomes the RHEOLOGY BULLETIN. Com- mencing with this issue, too, we resume the numbering of volumes, etc.. to agree with the old Journal of Rheology, Vol. -

Biological Materials: a Materials Science Approach✩
JOURNALOFTHEMECHANICALBEHAVIOROFBIOMEDICALMATERIALS ( ) ± available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jmbbm Review article Biological materials: A materials science approachI Marc A. Meyers∗, PoYu Chen, Maria I. Lopez, Yasuaki Seki, Albert Y.M. Lin University of California, San Diego, La Jolla, CA, United States ARTICLEINFO ABSTRACT Article history: The approach used by Materials Science and Engineering is revealing new aspects Received 25 May 2010 in the structure and properties of biological materials. The integration of advanced Received in revised form characterization, mechanical testing, and modeling methods can rationalize heretofore 20 August 2010 unexplained aspects of these structures. As an illustration of the power of this Accepted 22 August 2010 methodology, we apply it to biomineralized shells, avian beaks and feathers, and fish scales. We also present a few selected bioinspired applications: Velcro, an Al2O3PMMA composite inspired by the abalone shell, and synthetic attachment devices inspired by gecko. ⃝c 2010 Elsevier Ltd. All rights reserved. Contents 1. Introduction and basic components ............................................................................................................................................. 1 2. Hierarchical nature of biological materials ................................................................................................................................... 3 3. Structural biological materials..................................................................................................................................................... -

Polymers: a Historical Perspective
Journal & Proceedings of the Royal Society of New South Wales, vol. 152, part 2, 2019, pp. 242–250. ISSN 0035-9173/19/020242-09 Polymers: a historical perspective Robert Burford, FRSN Emeritus Professor, School of Chemical Engineering, UNSW Sydney Email: [email protected] Abstract This commissioned paper outlines the emergence of new forms of synthetics and plastics as our under- standing of polymer chemistry has advanced. Synopsis phenols and styrene are “polymerised” to olymers have been ubiquitous since form thermosets,1 including phenol for- simple gaseous molecules began to form maldehyde “Bakelite” thermosets, but are P 2 life-giving organic structures many millions also present in thermoplastics including of years ago. Today, we rely upon proteins polystyrene and related materials such as comprising twenty amino acids, as well as styrene acrylonitrile (SAN) and ABS.3 The DNA and RNA with many fewer nucleic manufacture of Bakelite is often viewed as acids. Similarly, many fibres and plants the birth of the synthetic polymer industry. comprise carbohydrate polymers: we and The enormous growth both in diversity and other animals use these and protein-based volume of thermoplastics is a feature of the polymers for our diet. Hence, organic earth’s 20th century, dismissively called the “plastics surface has an enormous diversity of natu- age.” Again, important but sometimes ser- rally occurring polymers, sometimes called endipitous discoveries are a feature of this macromolecules. period, but the associated large-scale produc- Today, these continue to feed and clothe tion introduced multinational corporations us, and much more, but the beginning of originating mainly in Europe, the US and man-made materials might be considered Japan. -

BS in Applied Physics (Optics Emphasis)
B.S. in Applied Physics (Optics Emphasis) 2016-2017: Option 1 - CWILT First Year Fall Credits Interim Credits Spring Credits PHY292 & 292D General Physics I and General Physics Lab *1 4 GES125 Introduction to the Creative Arts 4 PHY296 & PHY297 General Physics II and General Physics Lab II 4 CHE208 & 208D Accelerated General Chemistry and Accelerated General Chemistry Lab 4 MAT125 Calculus 2 4 GES106 Introduction to Liberal Arts 1 GES130 Christianity Western Culture 4 BIB101 Introduction to the Bible 3 GES110 College Writing 3 Mathematics (M) course 3 15 4 15 Second Year Fall Credits Interim Credits Spring Credits PHY302 & PHY303 Electronics and Electronics Lab 4 World Cultures (U) course 3 PHY312 & PHY313 Modern Physics and Modern Physics Lab 4 PHY352 & PHY353 Computer Methods in Physics and Engineering and Computer Methods in MAT223 Multivariable Calculus 3 Physics and Engineering Lab 4 COS205 Scientific Computing 3 MAT222 Differential Equations 3 PHY260 Careers in Engineering and Physics Seminar 1 THE201 Christian Theology 3 Nature of Persons (N) course 3 PEA100 Physical Wellness for Life 1 14 3 15 Third Year Fall Credits Interim Credits Spring Credits CHE113 & 113D General Chemistry I and General Chemistry I Lab 4 Contemporary Western Life and Thought (L) course 3 PHY365 Physics Research Seminar 1 PHY400 Electricity and Magnetism 4 PHY332 & PHY333 Optics and Optics Lab 4 Science, Technology, and Society (K) course 3 PHY440 Quantum Mechanics 4 Second Language (S) course2 4 Comparative Systems (G) course 3 Interpreting Biblical themes -

Materials Science
Materials Science Materials Science is an interdisciplinary field combining physics (fundamental laws of nature), chemistry (interactions of atoms) and biology (how life interacts with materials) to elucidate the inherent properties of basic and complex systems. This includes optical (interaction with light), electrical (interaction with charge), magnetic and structural properties of everyday electronics, clothing and architecture. The Materials Science central dogma follows the sequence: Structure—Properties—Design—Performance. This involves relating the nanostructure of a material to its macroscale physical and chemical properties. By understanding and then changing the structure, material scientists can create custom materials with unique properties. The goal of the materials science minor is to create a cross-disciplinary approach to fundamental topics in basic and applied physical sciences. Students will gain experience and perspectives from the disciplines of chemistry, physics and biology. The minor places a strong emphasis on current nanoscale research methods in addition to the basics of electronic, optical and mechanical properties of materials. Any student with an interest in pursuing the cross-disciplinary minor in materials science should consult with the coordinator of the minor. Students are encouraged to declare their participation in their sophomore year but no later than the end of the junior year. Students also should seek an adviser from participating faculty. Degree Requirements for the Minor General College requirements -

Fluorescence Correlation Spectroscopy in Polymer Science Cite This: RSC Adv.,2014,4, 2447 Ab Dominik Woll¨ *
Erschienen in: RSC Advances ; 4 (2014), 5. - S. 2447-2465 https://dx.doi.org/10.1039/C3RA44909B RSC Advances REVIEW View Article Online View Journal | View Issue Fluorescence correlation spectroscopy in polymer science Cite this: RSC Adv.,2014,4, 2447 ab Dominik Woll¨ * Fluorescence correlation spectroscopy (FCS) is a well-established technique for studying dynamic processes and interactions with minimal invasion into the corresponding system. Even though FCS has been mainly applied to biological systems, within the last 15 years an increasing number of studies in material sciences have appeared, demonstrating its enormous potential also for this field. Apart from investigations on colloidal systems, polymer science has benefited significantly from this technique. This review will summarize FCS studies on polymer systems and, in particular, focus on the diffusion of differently sized molecular and macromolecular probes in polymer solutions, classical and responsive polymer gels, polymer melts and glasses. It will be discussed how FCS can be used to determine Received 5th September 2013 translational and rotational diffusion in polymer solutions and at interfaces, scaling laws, micellization Accepted 5th November 2013 and aggregation and, to some extent, polymer structure including heterogeneity. Thus, FCS should be DOI: 10.1039/c3ra44909b considered a powerful complement to other methods for the investigation of polymer structure and www.rsc.org/advances dynamics. Polymers have emerged as the most important materials of the and only combining their strengths will allow us to gain a modern world. The reason for their success lies in the variety of consistent picture of polymers from the nanoscopic to the functions they can cover due to their tunable properties. -

Polymer Chemistry Degree Requirements Faculty Bachelor of Science Degree Petar R
Polymer Chemistry Degree requirements Faculty Bachelor of Science Degree Petar R. Dvornic, Ph.D., in Polymer Chemistry** Chair, Professor of Chemistry CORE SCIENCE COURSES (36 HOURS) CHEM-215: General Chemistry I (3 hours) Ram Gupta, Ph.D., and CHEM-216: General Chemistry I Lab (2 hours) Assistant Professor of Chemistry Pittsburg State University CHEM-225: General Chemistry II (3hours) and CHEM-226: General Chemistry II Lab (2 hours) Santimukul Santra, Ph.D., CHEM-235: Laboratory Safety & Compliance (1 hour) CHEM-325: Organic Chemistry I (3 hours) Assistant Professor of Chemistry and CHEM-326: Organic Chemistry Lab (2 hours) Jeanne Norton, Ph.D., Polymer CHEM-335: Organic Chemistry II (3 hours) and CHEM-336: Organic Chemistry II Lab (2 hours) Assistant Professor of MATH-150: Calculus I (5 hours) Plastics Engineering Technology PHYS-104: Engineering Physics I (4 hours) and PHYS-130: Elementary Physics Lab I (1 hour) Charles (Jody) Neef, Ph.D., Chemistry PHYS-105: Engineering Physics II (4 hours) Assistant Professor of Chemistry and PHYS-132: Engineering Physics Lab II (1 hour) POLYMER CHEMISTRY CORE COURSES (22-24 HOURS) Paul Herring, CHEM-360: Intro to Polymer Science & Technology (3 hours) Associate Professor of CHEM-611: Senior Review & Assessment (1 hour) CHEM-625: Polymer Synthesis & Characterizations (3 hours) Plastics Engineering Technology and CHEM-626: Polymer Synthesis & Characterizations Laboratory (2 hours) Bob Susnik, CHEM-680: Physical Properties of Polymers (3 hours) Professor of Plastics Engineering Technology CHEM-681: