Topics in Applied Physics; V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PHOTONICS and PLASMONICS SEMINAR – SPRING 2011 Unit Value: 1 Instructor: Prof
EE298‐5 PHOTONICS AND PLASMONICS SEMINAR – SPRING 2011 unit value: 1 instructor: Prof. Ivan Kaminow, [email protected] coordinator: Lea Barker, [email protected] class time: FRIDAYS, 11:00AM ‐ 12:30, 521 CORY HALL pre‐requisite: An interest in Photonics and/or Plasmonics. May be taken for credit and/or fun. website: http://inst.eecs.berkeley.edu/~ee298‐5/sp11/ plasmonics list: [email protected] This course is intended to give students at the advanced undergraduate or graduate level, and researchers, insight into current research based on a series of invited talks. SCHEDULE: 1/21 ‐ Prof. REUVEN GORDON, U. Victoria, Canada, “Challenging the Limits of Diffraction” Abstract: This talk will show ways of challenging three limits of optical diffraction. First, it will be shown how to focus light below the Abbe diffraction limit [1]. Next it will be shown how to squeeze light through small holes in a metal screen, allowing for 100% transmission in some cases, in contrast to Betheʹs aperture theory as found in textbooks [2,3]. This has interesting applications for biosensors. Finally, it will be shown how to optically trap nanoparticles with powers orders of magnitude smaller than required by conventional by Rayleigh scattering formulations [4], which has interesting applications for manipulating viruses and quantum dots. Theoretical and experimental results will be presented. (1) R. Gordon, ʺProposal for superfocusing at visible wavelengths using radiationless interference of a plasmonic array,ʺ Physical Review Letters, 102, 207402 (2009). (2) R. Gordon, A. G. Brolo, A. McKinnon, A. Rajora, B. Leathem, and K. L. Kavanagh, ʺStrong polarization in the optical transmission through elliptical nanohole arraysʺ Physical Review Letters, 92, 037401, (2004). -
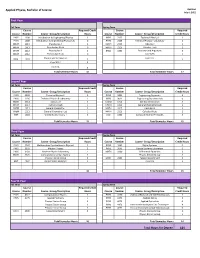
Physics, Applied Physics, B.S
Applied Physics, Bachelor of Science Updated July 6, 2021 First Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description Credit Hours PHYS 1308 Introduction to Engineering/Physics 3 PHYS 2325 Technical Physics I 3 PHYS 1008 Introduction to Engineering/Physics Lab 0 PHYS 2125 Technical Physics I Laboratory 1 MATH 2211 Precalculus A 2 MATH 2313 Calculus I 3 MATH 2011 Precalculus A Lab 0 MATH 2113 Calculus I Lab 1 MATH 2212 Precalculus B 2 ENGL 1302 Research and Argument 3 MATH 2012 Precalculus B Lab 0 Core S/BS 3 ENGL 1301 Rhetoric and Composition 3 Core LPC 3 Core SPCH 3 Core CA 3 Total Semester Hours 16 Total Semester Hours 17 Second Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description Credit Hours PHYS 2326 Technical Physics II 3 PHYS 3421 Engineering Dynamics 4 PHYS 2126 Technical Physics II Laboratory 1 PHYS 3021 Engineering Dynamics Lab 0 MATH 2314 Calculus II 3 CHEM 1312 General Chemistry II 3 MATH 2114 Calculus II Lab 1 CHEM 1112 General Chemistry II Lab 1 CHEM 1311 General Chemistry I 3 MATH 3315 Calculus III 3 CHEM 1111 General Chemistry I Lab 1 MATH 3115 Calculus III Lab 1 HIST 1301 United States History I 3 CSCI 1302 Computer Science Principles 3 Total Semester Hours 15 Total Semester Hours 15 Third Year Fall Term Spring Term Course Required Credit Course Required Course Number Course Group/Description Hours Course Number Course Group/Description -

Marko Lončar
MARKO LONČAR Harvard University http://nano-optics.seas.harvard.edu School of Engineering And Applied Sciences [email protected] Pierce Hall, 107C (617) 495-5798 29 Oxford Street Cambridge, MA 02138 Career History Harvard College Professor May 3, 2017 - present Harvard University Tiantsai Lin Professor of Electrical Engineering Jul. 1st, 2012 – present Harvard University Associate Professor of Electrical Engineering Jul. 1st, 2010 – Jun. 30th, 2012 Harvard University Assistant Professor of Electrical Engineering Jul. 1st, 2006 – Jun. 30th, 2010 Harvard University Postdoctoral Scholar in Applied Physics Oct. 1st, 2003 – Jun. 31st, 2006 Harvard University; Adviser: Federico Capasso Education Ph.D. in Electrical Engineering, California Institute of Technology 1998-2003 Thesis “Nanophotonics devices based on planar photonic crystals”. Adviser: Axel Scherer M.S. in Electrical Engineering, California Institute of Technology 1997-1998 Power electronics group. Adviser: Slobodan Ćuk Diploma in Electrical Engineering, University of Belgrade (R. Serbia) 1992-1997 Alfred P. Sloan Research Fellowship 02/16/2010 Awards and Recognitions NSF CAREER Award 2/1/2009 – 1/31/2014 Kavli Fellow (2013 US Kavli Frontiers of Science participant) November 2013 Levenson Prize for Excellence in Undergraduate Teaching, Harvard University 2011/12 academic year Fellow of Optical Society of America, Senior Member of IEEE and SPIE Research We study and engineer light-matter interaction in nanostructures, and apply these effects to develop novel and/or Interests better performing devices & systems. Examples include quantum photonics with diamond color centers, nonlinear nanophotonics (e.g. frequency combs, quantum wavelength conversion), behavior of light in composite metamaterials designed via topology optimization (inverse design), etc. Our efforts span a variety of optical materials (e.g. -

Rheology Bulletin
RHEOLOGY BULLETIN izoLvza §ei Publication of the SOCIETY OF RHEOLOGY Vol. XII No. 1 February 1941 « RHEOLOGY BULLETIN Published Quarterly by THE SOCIETY OF RHEOLOGY Dedicated to the Development of the Science of the Deformation and Flow of Matter 175 Fifth Avenue New York, N. Y. THE SOCIETY OF RHEOLOGY IS ONE OF THE FIVE FOUNDER SOCIETIES OF THE AMERICAN INSTITUTE OF PHYSICS A. STUART HUNTER, President WHEELER P. DAVEY, Editor Rayon Department Room 6, New Physics Pldg. E. I. du Pont de Nemours & Company The Pennsylvania State College Buffalo, New York State College, Pennsylvania J. H. DILLON, First Vice-President H. F. WAKEFIELD, Publishing Editor Firestone Tire & Rubber Company Bakelite Corporation Akron, Ohio Bloomfield, New Jersey R. H. EWELL, Second Vice-President H. R. LILLIE, Secretary-Treasurer Purdue University Corning Glass Works Lafayette, Indiana Corning, New York Non-member Subscriptions: $2.00 Annually Single Copies: 75c Apiece Adress All Communications to the Editor RHEOLOGY BULLETIN Vol. XII No. 1 TTdvra gel February 1941 TABLE OF CONTENTS Page Rheological News - 2 Report on Conference on "Viscosity" H. Mark, Polytechnic Institute of Brooklyn 3 Report of Committe on Definitions 6 Correlated Abstracts —« 12 Viscosity Flow of Metals Geological Applications Theory Plastics Contributed Papers The Viscosity of Helium II A. D. Misener, University of Toronto — 1 > The Transient Resilience of Some Fluids J. M. Kendall, Geophysical Corp 2( 1. The Leaflet Becomes The Bulletin. Commencing with this issue, the Rheology Leaflet becomes the RHEOLOGY BULLETIN. Com- mencing with this issue, too, we resume the numbering of volumes, etc.. to agree with the old Journal of Rheology, Vol. -

Materials Science and Engineering 1
Materials Science and Engineering 1 EN.515.603. Materials Characterization. 3 Credits. MATERIALS SCIENCE AND This course will describe a variety of techniques used to characterize the structure and composition of engineering materials, including metals, ENGINEERING ceramics, polymers, composites, and semiconductors. The emphasis will be on microstructural characterization techniques, including optical The Materials Science and Engineering Program for professionals allows and electron microscopy, x-ray diffraction, and acoustic microscopy. students to take courses that address current and emerging areas Surface analytical techniques, including Auger electron spectroscopy, critical to the development and use of biomaterials, electronic materials, secondary ion mass spectroscopy, x-ray photoelectron spectroscopy, structural materials, nanomaterials and nanotechnology, and related and Rutherford backscattering spectroscopy. Real-world examples of materials processing technologies. Students in this program gain an materials characterization will be presented throughout the course, advanced understanding of foundational concepts and are exposed to including characterization of thin films, surfaces, interfaces, and single the latest research that is driving materials-related advances. crystals. Courses are offered at the Applied Physics Laboratory, the Homewood EN.515.605. Electrical, Optical and Magnetic Properties. 3 Credits. campus, and online. An overview of electrical, optical and magnetic properties arising from the fundamental electronic and atomic structure of materials. Continuum materials properties are developed through examination of microscopic Program Committee processes. Emphasis will be placed on both fundamental principles and James Spicer, Program Chair applications in contemporary materials technologies.Course Note(s): Principal Professional Staff Please note that this 515 course is also listed as a 510 course in the full- JHU Applied Physics Laboratory time program. -

BS in Applied Physics (Optics Emphasis)
B.S. in Applied Physics (Optics Emphasis) 2016-2017: Option 1 - CWILT First Year Fall Credits Interim Credits Spring Credits PHY292 & 292D General Physics I and General Physics Lab *1 4 GES125 Introduction to the Creative Arts 4 PHY296 & PHY297 General Physics II and General Physics Lab II 4 CHE208 & 208D Accelerated General Chemistry and Accelerated General Chemistry Lab 4 MAT125 Calculus 2 4 GES106 Introduction to Liberal Arts 1 GES130 Christianity Western Culture 4 BIB101 Introduction to the Bible 3 GES110 College Writing 3 Mathematics (M) course 3 15 4 15 Second Year Fall Credits Interim Credits Spring Credits PHY302 & PHY303 Electronics and Electronics Lab 4 World Cultures (U) course 3 PHY312 & PHY313 Modern Physics and Modern Physics Lab 4 PHY352 & PHY353 Computer Methods in Physics and Engineering and Computer Methods in MAT223 Multivariable Calculus 3 Physics and Engineering Lab 4 COS205 Scientific Computing 3 MAT222 Differential Equations 3 PHY260 Careers in Engineering and Physics Seminar 1 THE201 Christian Theology 3 Nature of Persons (N) course 3 PEA100 Physical Wellness for Life 1 14 3 15 Third Year Fall Credits Interim Credits Spring Credits CHE113 & 113D General Chemistry I and General Chemistry I Lab 4 Contemporary Western Life and Thought (L) course 3 PHY365 Physics Research Seminar 1 PHY400 Electricity and Magnetism 4 PHY332 & PHY333 Optics and Optics Lab 4 Science, Technology, and Society (K) course 3 PHY440 Quantum Mechanics 4 Second Language (S) course2 4 Comparative Systems (G) course 3 Interpreting Biblical themes -

Applied Physics Certificate
SOUTH DAKOTA BOARD OF REGENTS ACADEMIC AFFAIRS FORMS New Certificate UNIVERSITY: SDSU TITLE OF PROPOSED CERTIFICATE: Applied Physics INTENDED DATE OF IMPLEMENTATION: 2022-2023 Academic Year PROPOSED CIP CODE: 40.0801 UNIVERSITY DEPARTMENT: Grad Study Physics BANNER DEPARTMENT CODE: SGPH UNIVERSITY DIVISION: Graduate School BANNER DIVISION CODE: 3G ~ Please check this box to confirm that: • The individual preparing this request has read AAC Guideline 2.7, which pertains to new certificate requests, and that this request meets the requirements outlined in the guidelines. • This request will not be posted to the university website for review of the Academic Affairs Committee until it is approved by the Executive Director and Chief Academic Officer. University Approval To the Board ofRegents and the Executive Director: I certify that I have read this proposal, that I believe it to be accurate, and that it has been evaluated and approved as provided by university polic Institutional Approval Signature Presi 'f!nt or ChiefAcademic Officer ofthe University 1. Is this a graduate-level certificate or undergraduate-level certificate? Undergraduate Certificate Graduate Certificate ~ 2. What is the nature/ purpose of the proposed certificate? Please include a brief (1-2 sentence) description of the academic field in this certificate. South Dakota State University (SDSU) requests authorization to offer a graduate certificate in Applied Physics. The proposed Applied Physics Certificate will provide knowledge and practical experience for students to have an advanced experience with health physics, nuclear physics, or condensed matter physics. Individuals who complete this certificate will be well prepared to advance in their career paths in applied areas of physics, including laboratory management. -

Fall 2010 Applied Quantum Mechanics for Engineers (3) Instructor: Prof
ECE 350 / 450 - Fall 2010 Applied Quantum Mechanics for Engineers (3) Instructor: Prof. Nelson Tansu ECE & COT, Lehigh University Office: Sinclair Laboratory Room 218 Lab: MOCVD Laboratory, Room 251 Sinclair Lab. 7 Asa Drive, Bethlehem, PA 18015 Email: [email protected] www.ece.lehigh.edu/~tansu Required Reading Textbook: 1. Nelson Tansu, Applied Quantum Mechanics for Engineers, Draft Version (2004- 2010). We will cover approximately 10-11 chapters in the required reading, with approximately 3-4 additional chapters for your own perusal. The required text consists of approximately 14 chapters. Coverage for Fall 2007 semester: Chapters 1-9, 12, 13. Optional materials: 10, 11, 14. Other Additional References or Readings: 1. Peter L. Hagelstein, Stephen D. Senturia, and Terry P. Orlando, Introductory Applied Quantum and Statistical Mechanics, Wiley (2004). 2. A. F. J. Levi, Applied Quantum Mechanics (2nd Edition), Cambridge (2006). 3. D. A. B. Miller, Quantum Mechanics for Scientists and Engineers, Cambridge (2008). 4. Richard Liboff, Introductory Quantum Mechanics, 4th edition, Addison Wesley (2003). 5. Jasprit Singh, Quantum Mechanics: Fundamentals and Applications to Technology, Wiley (1996). 6. Herbert Kroemer, Quantum Mechanics: For Engineering, Materials Science, and Applied Physics, Prentice Hall (1994). Course Abstract: This course covers the fundamentals of quantum mechanics, and applications of quantum mechanics to engineering and applied physics problems. Classical physics is an approximation of quantum physics, in the case that the dimensions of interest are many orders magnitude larger than those of atoms. Though classical physics can explain and approximate some of the phenomena quite accurately, in reality, our world is a quantum world and it should only be described with quantum physics. -

Equilibrium Thermodynamics: Methods and Results
Advanced Materials Science Research Optimization problems in non - equilibrium thermodynamics: Methods and results Ivan Sukin Abstarct Program Systems Institute of Russian Thermodynamics estimates for ultimate capacity of various systems exist for a long time. Such estimates Academy of Sciences, Russian Federation include Carnot effi ciency and Gibbs minimum work of separation. These classical estimates do not take Biography irreversibility into account. Sources of the irreversibility are heat and mass transfer phenomena. For some processes, such as the heat transfer, classical estimates simple do not make any sense, since the process Ivan Sukin has expertise in process con- is suffi ciently irreversible. These problems lead to the creation of the fi nite-time thermodynamics, which trol and optimization. His fi eld of inter- est is the thermodynamics, control and divides the system into union internally reversible subsystems. The interaction of these subsystems optimization of separation processes. He is irreversible and leads to the entropy generation. One could solve optimization problems within this works within the Anatoly Tsirlin’s school framework. The general approach is following: 1. Write the balance equations. These are mass, energy and of fi nite-time thermodynamics that has its roots within the fi rst works in this fi entropy balances. The entropy balance equation contains the entropy generation. 2. Minimize the entropy eld. The passion of sukin is the distillation generation for given system structure and fl uxes. 3.Construct the feasible set of the system, substituting optimal control. the minimum entropy generation into balance equations. The talk summarizes solutions of the above- mentioned problems for systems of heat and mass transfer, chemical reactions, separation processes, heating and cooling engines. -

Information for Engineering Students
Physics Undergraduate Degree Programs for Engineering Students The Physics Department has multiple degree programs for undergraduates whose first major is in engineering, but who would like to double major in physics. The degree programs are BA in physics BS in physics / BS in astronomy-physics The BA in physics is designed for students interested in physics and planning to enter other professional fields like engineering, business, education, law, and medicine. The BS, and BS in astronomy-physics are designed for graduate study in physics, other sciences, or in engineering, and for those planning to enter a job in a scientific/technical field. The requirements for the BA degree with substitutions as usual for engineering students are: Required course: Regular Engineering School substitute course: MATH 1320 Calculus II APMA 1110 Single Variable Calculus II MATH 2310 Calculus III APMA 2120 Multivariable Calculus MATH 3255 Diff. Eq. APMA 2130 Applied Diff. Eq. PHYS 1425 Intro. Phys. for Eng. I PHYS 1429 Intro. Phys. I Workshop PHYS 2415 Intro. Phys. for Eng. II Electrical and Computer Engineering PHYS 2419 Intro. Phys. II Workshop Students can substitute PHYS 2415 and PHYS 2419 with ECE3209 (Electromagnetic Fields). PHYS 2620 Modern Physics PHYS 27201 Problems Four electives 1 This is a new requirement, for students in class of 2025 and beyond Note that only the courses in the last three rows are in addition to what Engineering School requires anyway. Electives can be chosen from PHYS 2660 (Fundamentals of Scientific Computing) and/or -

Optics at the Applied Physics Laboratory
_____________________________________________________ THEMEARTICLES WILLIAM J. TROPF OPTICS AT THE APPLIED PHYSICS LABORATORY Work in optics at the Applied Physics Laboratory has described in the following paragraphs. References 1 to steadily grown over the past decade, and has become 24 are articles on optics from previous issues of the Tech a major discipline that rivals the traditional APL special nical Digest. Those past articles, along with the articles ties in microwaves and acoustics. Optical activities at in this issue, present examples of APL work in optics. APL include areas as diverse as research, sensor evalu ation, and optical techniques as applied to medical, Fleet Systems Department space, military, and oceanographic tasks. Optical work in the Fleet Systems Department focuses This issue of the Johns Hopkins APL Technical Di primarily on performance of detection, tracking, I guid gest reviews current and recent tasks in optics at APL. ance, 7 and communication4 systems for the Navy, as Harris begins with an introductory article on optical de well as basic research in propagation, 23 material prop sign that is intended to acquaint the nonspecialist with erties, 24 device characterization, mensuration, and re the optical design process, available tools, and typical mote sensing. Research activities in the department in APL products. clude optical signal processing, property measurements, Several articles look at different facets of optical tech remote sensing, and biomedical instrumentation develop nology. Thomas and Joseph describe a process for char ment. The department has a complete optical design and acterizing the optical properties of transparent materials measurement capability that supports instrument devel through a combination of theory, models, and measure opment and performance evaluation throughout APL. -

Applied Physics BA
QUEENS COLLEGE Applied Physics BA FOUR-YEAR ACADEMIC PLAN 12 Required Core Credits 18 Flexible Core Credits 12 College Option Credits 60 Major Credits 18 Elective Credits Total 120 This 4-year academic plan is designed to help freshmen entering Queens College in Fall 2018 plan their academic career and ensure that they complete all requirements for graduation in a timely fashion. All other students should consult their academic and department advisors to chart their own 4-year academic plans. Students should note that course pre-requisite/s and co-requisite/s are strictly enforced and they should regularly meet with their department advisors to identify their specific major/minor requirements and entrance and maintenance criteria (if applicable) for successful completion of their degree. QUEENS COLLEGE Applied Physics BA FOUR-YEAR ACADEMIC PLAN Freshman FALL SPRING English Composition I (EC1) 3 credits English Composition II (EC2) 3 credits MATH 151 (MQR) 4 credits PHYS 1454 & 1451 (LPS) 5 credits Calculus I Principles of Physics I World Cultures & Global Issues (WCGI) 3 credits MATH 152 4 credits U.S Experience in its Diversity (USED) 3 credits Calculus II Creative Expression (CE) 3 credits Individual and Society (IS) 3 credits Fall total credits 16 credits Spring total credits 15 credits Sophomore FALL SPRING PHYS 1464 & 1461 (SCI) 5 credits PHYS 225 4 credits Principles of Physics II Solid State Electronics MATH 201 4 credits PHYS 235 2 credis Calculus III Classical Physics LAB An Additional Flexible Core 3 credits Scientific World (SW) 3 credits One Writing Intensive Unit (W) 3 credits College Option Literature (LIT + W) 3 credits With Writing Intensive Unit* Fall total credits 15 credits College Option Language (LANG) 4 credits Spring total credits 16 credits General Education requirements may be taken in any order if the pre-requisite requirement(s) is/are satisfied.