Is the Metrial Gland Really a Gland?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -
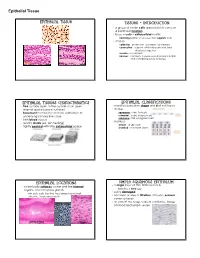
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Squamous Epithelium in the Human Thyroid Gland
J Clin Pathol: first published as 10.1136/jcp.19.4.384 on 1 July 1966. Downloaded from J. clin. Path. (1966), 19, 384. Squamous epithelium in the human thyroid gland J. N. HARCOURT-WEBSTER1 From the Department ofPathology, University ofEdinburgh SYNOPSIS Four cases are reported in each of which squamous epithelium was an incidental finding in surgically excised thyroid gland tissue. The occasional thyroid cyst lined throughout by squamous cells probably represents a persistent ultimo-branchial body, but the evidence indicates that the usual source of such cells in this gland is metaplasia of the follicular epithelium. An explanation is offered for the infrequency of this transformation in the thyroid, despite the frequent occurrence of the changes which predispose to epithelial metaplasia at other sites. There is no evidence to suggest that squamous cells arising in this gland by either of these means have any sinister significance. Squamous epithelium was first described in a human iodine uptake studies were normal but serological anti- thyroid gland by Nicholson (1922); he attributed body tests were positive (ADT: +ve on second day; this finding to metaplasia of the follicular epithelium TCH: 1/250,000; CFT: 1/250). The provisional diagnosis was Hashimoto's disease; a biopsy was taken from the induced by severe chronic inflammatory and fibrotic right lobe with a wedge resection of the isthmus. changes in the gland. Epithelial metaplasia occurs in Histology Sections of the rock-hard, pale fawn tissue response to altered function or at least as the result show abundant, interwoven, thick bands of hyaline col- of altered environment (Boyd, 1961). -

Mixed Type Adrenal Cyst: a Case Report
Eastern Journal of Medicine 20 (2015) 163-166 Case Report Mixed type adrenal cyst: A case report Gulay Buluta,*, Mehmet Deniz Bulutb, Deniz Yilmaza, Sebahattin Celikc, Nursen Toprakb aDepartment of Pathology, Yuzuncu Yil University Faculty of Medicine, Van, Turkey bDepartment of Radiology, Van Research and Training Hospital, Van, Turkey cDepartment of General Surgery, Van Research and Training Hospital, Van, Turkey Abstract. Adrenal cysts are rare lesions that usually progress asymptomatically. They are often determined post- mortem. Our case was a 50-year old-male who had presented to our clinic with upper right quadrant pain. The computed tomography demonstrated a cystic mass in the adrenal gland. No abnormalities were determined in the laboratory tests. According to the histopathological and immunohistochemical examination, the cyst was evaluated as a mixed-type adrenal cyst, the wall of which was covered with endothelium, mesothelium and cubic epithelium. We believed that the case was worth presenting, since it was the first diagnosed mixed-type adrenal cyst in the literature. Key words: Mixed-type adrenal cyst, endothelial cyst, epithelial cyst, mesothelial cyst pain. There was no history of hypertension or 1. Introduction trauma in the past. Per abdominal examination Adrenal cysts are rarely encountered clinical showed a soft abdomen; no mass was felt. entities. Their incidence has been reported from Haemogram was normal. A complete endocrine 0.06%-0.18% in autopsy series (1). In 1966, workup including the estimation of urinary Foster put forth a classification based on the metanephrines, vanillylmandelic acid and free histopathology of 220 adrenal cysts into parasitic cortisol did not show any hormonal cysts (7%), epithelial cysts (9%), pseudocysts hypersecretion. -

Squamous Epithelium Are Thin, Which Allows for the Rapid Passage of Substances Through Them
Chapter 2 1st Prof. Anatomy Arsalan (Lecturer Department of Pharmacy University of Peshawar) Tissue is an aggregation of similar cells and their products that perform same function. There are four principal types of tissues in the body: ❑ epithelial tissue: covers body surfaces, lines body cavities and ducts and forms glands ❑ connective tissue: binds, supports, and protects body parts ❑ muscle tissue: produce body and organ movements ❑ nervous tissue: initiates and transmits nerve impulses from one body part to another • Epithelial tissues cover body and organ surfaces, line body cavities and lumina and forms various glands • Derived from endoderm ,ectoderm, and mesoderm • composed of one or more layers of closely packed cells • Perform diverse functions of protection, absorption, excretion and secretion. Highly cellular with low extracellular matrix Polar – has an apical surface exposed to external environment or body cavity, basal layer attached to underlying connective tissue by basement membrane and lateral surfaces attached to each other by intercellular junctions Innervated Avascular – almost all epithelia are devoid of blood vessels, obtain nutrients by diffusion High regeneration capacity Protection: Selective permeability: in GIT facilitate absorption, in kidney facilitate filtration, in lungs facilitate diffusion. Secretions: glandular epithelium form linings of various glands, involved in secretions. Sensations: contain some nerve endings to detect changes in the external environment at their surface Epithelium rests on connective tissue. Between the epithelium and connective tissue is present the basement membrane which is extracellular matrix made up of protein fibers and carbohydrates. Basement membrane attach epithelium to connective tissue and also regulate movement of material between epithelium and connective tissue Epithelial cells are bound together by specialized connections in the plasma membranes called intercellular junctions . -

HISTOLOGY DRAWINGS Created by Dr Carol Lazer During the Period 2000-2005
HISTOLOGY DRAWINGS created by Dr Carol Lazer during the period 2000-2005 INTRODUCTION The first pages illustrate introductory concepts for those new to microscopy as well as definitions of commonly used histology terms. The drawings of histology images were originally designed to complement the histology component of the first year Medical course run prior to 2004. They are sketches from selected slides used in class from the teaching slide set. These labelled diagrams should closely follow the current Science courses in histology, anatomy and embryology and complement the virtual microscopy used in the current Medical course. © Dr Carol Lazer, April 2005 STEREOLOGY: SLICING A 3-D OBJECT SIMPLE TUBE CROSS SECTION = TRANSVERSE SECTION (XS) (TS) OBLIQUE SECTION 3-D LONGITUDINAL SECTION (LS) 2-D BENDING AND BRANCHING TUBE branch off a tube 2 sections from 2 tubes cut at different angles section at the beginning 3-D 2-D of a branch 3 sections from one tube 1 section and the grazed wall of a tube en face view = as seen from above COMPLEX STRUCTURE (gland) COMPOUND ( = branched ducts) ACINAR ( = bunches of secretory cells) GLAND duct (XS =TS) acinus (cluster of cells) (TS) duct and acinus (LS) 3-D 2-D Do microscope images of 2-D slices represent a single plane of section of a 3-D structure? Do all microscope slides show 2-D slices of 3-D structures? No, 2-D slices have a thickness which can vary from a sliver of one cell to several cells deep. No, slides can also be smears, where entire cells With the limited depth of field of high power lenses lie on the surface of the slide, or whole tissue it is possible to focus through the various levels mounts of very thin structures, such as mesentery. -

Terminologia Anatomica and Its Practical Usage: Pitfalls and How to Avoid Them
CORE Metadata, citation and similar papers at core.ac.uk Provided by Via Medica Journals ONLINE FIRST This is a provisional PDF only. Copyedited and fully formatted version will be made available soon. ISSN: 0015-5659 e-ISSN: 1644-3284 Terminologia Anatomica and its practical usage: pitfalls and how to avoid them Authors: Piotr Paweł Chmielewski, Zygmunt Antoni Domagała DOI: 10.5603/FM.a2019.0086 Article type: REVIEW ARTICLES Submitted: 2019-06-29 Accepted: 2019-07-10 Published online: 2019-07-29 This article has been peer reviewed and published immediately upon acceptance. It is an open access article, which means that it can be downloaded, printed, and distributed freely, provided the work is properly cited. Articles in "Folia Morphologica" are listed in PubMed. Powered by TCPDF (www.tcpdf.org) Terminologia Anatomica and its practical usage: pitfalls and how to avoid them Running title: New Terminologia Anatomica and its practical usage Piotr Paweł Chmielewski, Zygmunt Antoni Domagała Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University Address for correspondence: Dr. Piotr Paweł Chmielewski, PhD, Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University, 6a Chałubińskiego Street, 50-368 Wrocław, Poland, e-mail: [email protected] ABSTRACT In 2016, the Federative International Programme for Anatomical Terminology (FIPAT) tentatively approved the updated and extended version of anatomical terminology that replaced the previous version of Terminologia Anatomica (1998). This modern version has already appeared in new editions of leading anatomical atlases and textbooks, including Netter’s Atlas of Human Anatomy, even though it was originally available only as a draft and the final version is different. -

Mesothelium and Malignant Mesothelioma
Journal of Developmental Biology Review Mesothelium and Malignant Mesothelioma Emilye Hiriart, Raymond Deepe and Andy Wessels * Department of Regenerative Medicine and Cell Biology, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC 29425, USA; [email protected] (E.H.); [email protected] (R.D.) * Correspondence: [email protected]; Tel.: +1-843-792-8183 Received: 4 March 2019; Accepted: 5 April 2019; Published: 8 April 2019 Abstract: The mesothelium is an epithelial structure derived from the embryonic mesoderm. It plays an important role in the development of a number of different organs, including the heart, lungs, and intestines. In this publication, we discuss aspects of the development of the mesothelium, where mesothelial structures can be found, and review molecular and cellular characteristics associated with the mesothelium. Furthermore, we discuss the involvement of the mesothelium in a number of disease conditions, in particular in the pathogenesis of mesotheliomas with an emphasis on malignant pleural mesothelioma (MPM)—a primary cancer developing in the pleural cavity. Keywords: mesothelium; development; malignant; mesothelioma; cancer 1. Introduction Malignant mesothelioma is a neoplasm that originates from mesothelial cells lining the body cavities, including the pleura, peritoneum, pericardium, and tunica vaginalis. The majority of malignant mesothelioma cases are mesotheliomas that develop in the pleural cavity. They are known as malignant pleural mesothelioma (MPM) and comprise 70–90% of all reported cases of malignant mesothelioma [1,2]. The other cases typically arise in the peritoneum [3], while the pericardium is rarely affected [4]. In this review we will briefly discuss the origin of the mesothelial structures, provide a succinct overview of molecular mechanisms involved in their development, and address aspects of the etiology and pathogenesis of mesotheliomas. -

Frequency Vs. Interval
Frequency vs. Interval • Milking Frequency – 2 times/day (2X) or 3 times/day (3X) • Milking Interval – 12 hours, 8 hours, etc. • How often do cows want to be milked? Frequency vs. Interval What are the advantages and disadvantages of more frequent milkings? Chemotactic agents: attract PMN into tissues & milk! • Alveoli • Basic milk-producing unit • Lined with epithelial cells • Phagocyte • Cell that engulfs and absorbs bacteria • PMN • Polymorphonuclear neutrophil • First line of defense against invading pathogens during mastitis • Majority cell type accounting for SCC • Macrophages, lymphocytes • Chemotaxis • Movement of an organism in response to a chemical stimulus • Somatic cells and bacteria move according to chemicals in their environment • Where and why would they be moving? • What is a common example of chemotaxis unrelated to milk secretion? Altered Composition During Mastitis Somatic cell counts (SCC) Na, Cl, whey protein (e.g., serum albumin, Ig) lactose, casein, K, α-lactalbumin Altered Composition During Mastitis • Lactose • Synthesis is decreased • Casein • Proteolysis • Proteolytic enzymes from leukocytes and bacteria • Milk fat • Susceptibility of milk fat globule membranes to the action of lipases, resulting in breakdown of triglycerides. Altered Composition During Mastitis • Na+, Cl-, K+ • Electrical potential across apical membrane disrupted • This is the basis of the electrical conductivity methods of detecting mastitis • https://www.youtube.com/watch?v=P-imDC1txWw • Polymorphonuclear neutrophils (PMNs) • Mastitis causes chemotaxis of the cells into the tissue and disruption of epithelial tight junctions • This is the basis of many mastitis detection methods • Albumin, immunoglobulins • Enter the milk via disrupted tight junctional complexes PHYLOGENY & ONTOGENY Phylogeny – the evolutionary development of any animal species (related to mammary gland development) Class Mammalia: Monotremes I. -

57 Lacrimal Gland
Lacrimal Gland Lacrimal glands are well-developed, compound tubuloalveolar glands of the serous type, located in the superior temporal region of the orbit. Each gland consists of several separate glandular units that empty into the conjunctival sac via 6 to 12 ducts. The secretory units consist of tall columnar cells with large, pale secretory granules. Numerous myoepithelial cells lie between the bases of the secretory cells and their basal lamina. The ducts are lined at first by simple cuboidal epithelium, which becomes stratified columnar in the larger ducts. Small accessory lacrimal glands can occur in the inner surface of the eyelid. Accessory lacrimals occur in the fornix of the conjunctiva (glands of Krause) and just above the tarsal plate (glands of Wolfring). Secretions of the lacrimal gland moisten, lubricate, and flush the anterior surface of the eye and the interior of the eyelids. Excess tears collect at a medial expansion of the conjunctival sac, which is drained by the lacrimal duct to a lacrimal sac at the medial corner of each eye. The lacrimal duct is lined by stratified squamous epithelium. The lacrimal sac and the nasolacrimal duct, which drains the sac into the inferior meatus of the nasal cavity, are lined by pseudostratified columnar epithelium. The remainder of their walls consists of dense connective tissue. The tear film ensures an optimal refractive surface and provides a mechanical and antimicrobial barrier on the corneal surface. The lipid component originates from Meibomian glands and forms the superficial layer of the tear film. The aqueous component contains water, electrolytes, a large variety of proteins, peptides and glycoproteins (mucins) secreted by the lacrimal glands.