A Brief History of Nuclear Astrophysics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Almost Forgotten Anniversaries in 2019 Introduction
Almost Forgotten Anniversaries in 2019 Katharina Lodders Department of Earth and Planetary Sciences and Mc Donnell Center for the Space Sciences, Campus Box 1169, Washington University, Saint Louis MO 63130, USA Keywords: history, chemical elements, abundances Abstract: As we celebrate the International Year of the Periodic Table, the 50th anniversary of Apollo 11 and the meteorite falls of Allende and Murchison in 1969, other noteworthy science events with round birthdays seem to be overlooked and almost forgotten Several scientific organizations celebrate the birthdays of their foundation; and key events and discoveries related to meteoritics, astronomy, geo- and cosmochemistry, and nuclear sciences can be commemorated this year, including the anniversaries of the discoveries of eleven chemical elements, and the advancements of our knowledge of the elemental and isotopic abundances. Introduction. Introduction The 150th anniversary of the discovery of the periodic system of the elements by Dmitri Ivanovich Mendeleev (8 Feb. 1834 – 2 Feb. 1907) and independently by Julius Lothar Meyer (19 August 1830 – 11 April 1895) is the reason for celebrating the International Year of the Periodic Table in 2019. Not only that, but several scientific organizations celebrate the birthdays of their foundation: The Astronomical Society of the Pacific (1889), The American Astronomical Society (1899), the American Geophysical Union (1919), the Mineralogical Society of America (1919), and the International Astronomical Union IAU (1919). The anniversaries in 2019 give us reasons to reflect on the major impacts of space exploration. In 1969, the first men landed on the moon and Apollo 11 safely returned with lunar rocks for study. The same year was blessed by the fall of the important carbonaceous chondrites Allende and Murchison. -

George De Hevesy in America
Journal of Nuclear Medicine, published on July 13, 2019 as doi:10.2967/jnumed.119.233254 George de Hevesy in America George de Hevesy was a Hungarian radiochemist who was awarded the Nobel Prize in Chemistry in 1943 for the discovery of the radiotracer principle (1). As the radiotracer principle is the foundation of all diagnostic and therapeutic nuclear medicine procedures, Hevesy is widely considered the father of nuclear medicine (1). Although it is well-known that he spent time at a number of European institutions, it is not widely known that he also spent six weeks at Cornell University in Ithaca, NY, in the fall of 1930 as that year’s Baker Lecturer in the Department of Chemistry (2-6). “[T]he Baker Lecturer gave two formal presentations per week, to a large and diverse audience and provided an informal seminar weekly for students and faculty members interested in the subject. The lecturer had an office in Baker Laboratory and was available to faculty and students for further discussion.” (7) There is also evidence that, “…Hevesy visited Harvard [University, Cambridge, MA] as a Baker Lecturer at Cornell in 1930…” (8). Neither of the authors of this Note/Letter was aware of Hevesy’s association with Cornell University despite our longstanding ties to Cornell until one of us (WCK) noticed the association in Hevesy’s biographical page on the official Nobel website (6). WCK obtained both his undergraduate degree and medical degree from Cornell in Ithaca and New York City, respectively, and spent his career in nuclear medicine. JRO did his nuclear medicine training at Columbia University and has subsequently been a faculty member of Weill Cornell Medical College for the last eleven years (with a brief tenure at Memorial Sloan Kettering Cancer Center (affiliated with Cornell)), and is now the program director of the Nuclear Medicine residency and Chief of the Molecular Imaging and Therapeutics Section. -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

Stephan Hruszkewycz Tuesday, September 25 • 4 Pm • Tech L211
THE MATERIALS SCIENCE AND ENGINEERING DEPARTMENT COLLOQUIUM SERIES PRESENTS: Stephan Hruszkewycz Assistant Physicist, Argonne National Laboratory Opportunities for materials science with coherent x-ray diffraction imaging Recent progress in 3D coherent x-ray diffraction imaging methods can enable high resolution structural imaging of nano-structured crystalline materials under operating conditions. In this talk, I discuss developments in Bragg coherent diffraction imaging (BCDI) that aim to broaden the envelope of materials science problems that can be addressed with the technique. Following an introduction of the basic principles of the method, two specific topics will be discussed: 1) BCDI at high x-ray energies that provide dramatic penetrating ability, 2) Bragg ptychography that enable imaging of targeted sub- volumes of a crystal. Both approaches will be discussed in the context of materials science problems that can be addressed in-situ at next-generation synchrotron storage rings including the Upgraded Advanced Photon Source project now underway at Argonne National Laboratory. Stephan Hruszkewycz is a staff scientist in the Materials Science Division at Argonne National Laboratory. His research focuses on developing and using coherent x-ray scattering techniques to interrogate nanoscale materials structure and dynamics under working conditions to reveal structure-property relationships. Currently he is using strain- sensitive coherent Bragg diffraction to image subtle strain fields in nanoscale crystals for photonic and quantum information applications. These research thrusts are pursued at high-brightness synchrotron sources with state-of-the-art coherence-preserving beamlines, including those at the Advanced Photon Source, NSLS-II, and LCLS, and aim to broaden the applicability of coherent diffraction imaging within both the broader materials science community. -

Von Richthofen, Einstein and the AGA Estimating Achievement from Fame
Von Richthofen, Einstein and the AGA Estimating achievement from fame Every schoolboy has heard of Einstein; fewer have heard of Antoine Becquerel; almost nobody has heard of Nils Dalén. Yet they all won Nobel Prizes for Physics. Can we gauge a scientist’s achievements by his or her fame? If so, how? And how do fighter pilots help? Mikhail Simkin and Vwani Roychowdhury look for the linkages. “It was a famous victory.” We instinctively rank the had published. However, in 2001–2002 popular French achievements of great men and women by how famous TV presenters Igor and Grichka Bogdanoff published they are. But is instinct enough? And how exactly does a great man’s fame relate to the greatness of his achieve- ment? Some achievements are easy to quantify. Such is the case with fighter pilots of the First World War. Their achievements can be easily measured and ranked, in terms of their victories – the number of enemy planes they shot down. These aces achieved varying degrees of fame, which have lasted down to the internet age. A few years ago we compared1 the fame of First World War fighter pilot aces (measured in Google hits) with their achievement (measured in victories); and we found that We can estimate fame grows exponentially with achievement. fame from Google; Is the same true in other areas of excellence? Bagrow et al. have studied the relationship between can this tell us 2 achievement and fame for physicists . The relationship Manfred von Richthofen (in cockpit) with members of his so- about actual they found was linear. -
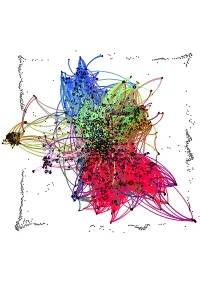
Network Map of Knowledge And
Humphry Davy George Grosz Patrick Galvin August Wilhelm von Hofmann Mervyn Gotsman Peter Blake Willa Cather Norman Vincent Peale Hans Holbein the Elder David Bomberg Hans Lewy Mark Ryden Juan Gris Ian Stevenson Charles Coleman (English painter) Mauritz de Haas David Drake Donald E. Westlake John Morton Blum Yehuda Amichai Stephen Smale Bernd and Hilla Becher Vitsentzos Kornaros Maxfield Parrish L. Sprague de Camp Derek Jarman Baron Carl von Rokitansky John LaFarge Richard Francis Burton Jamie Hewlett George Sterling Sergei Winogradsky Federico Halbherr Jean-Léon Gérôme William M. Bass Roy Lichtenstein Jacob Isaakszoon van Ruisdael Tony Cliff Julia Margaret Cameron Arnold Sommerfeld Adrian Willaert Olga Arsenievna Oleinik LeMoine Fitzgerald Christian Krohg Wilfred Thesiger Jean-Joseph Benjamin-Constant Eva Hesse `Abd Allah ibn `Abbas Him Mark Lai Clark Ashton Smith Clint Eastwood Therkel Mathiassen Bettie Page Frank DuMond Peter Whittle Salvador Espriu Gaetano Fichera William Cubley Jean Tinguely Amado Nervo Sarat Chandra Chattopadhyay Ferdinand Hodler Françoise Sagan Dave Meltzer Anton Julius Carlson Bela Cikoš Sesija John Cleese Kan Nyunt Charlotte Lamb Benjamin Silliman Howard Hendricks Jim Russell (cartoonist) Kate Chopin Gary Becker Harvey Kurtzman Michel Tapié John C. Maxwell Stan Pitt Henry Lawson Gustave Boulanger Wayne Shorter Irshad Kamil Joseph Greenberg Dungeons & Dragons Serbian epic poetry Adrian Ludwig Richter Eliseu Visconti Albert Maignan Syed Nazeer Husain Hakushu Kitahara Lim Cheng Hoe David Brin Bernard Ogilvie Dodge Star Wars Karel Capek Hudson River School Alfred Hitchcock Vladimir Colin Robert Kroetsch Shah Abdul Latif Bhittai Stephen Sondheim Robert Ludlum Frank Frazetta Walter Tevis Sax Rohmer Rafael Sabatini Ralph Nader Manon Gropius Aristide Maillol Ed Roth Jonathan Dordick Abdur Razzaq (Professor) John W. -

MEMOIRS Row Standing on the Stern, Venetian Style, from the Lido, Where I Lived, to the School in Venice, Where I Studied
BRUNO BENEDETTO ROSSI April 13, 1905–November 21, 1993 BY GEORGE W. CLARK The initial motivation of the experiment which led to this discovery [of Sco X-1] was a subconscious feeling for the inexhaustible wealth of nature, a wealth that goes far beyond the imagination of man. That feeling was possi- bly generated by experiences in my previous work on cosmic rays; more likely it was inborn and was the reason why, as a young man, I went into the field of cosmic rays. In any case, whenever technical progress opened a new window into the surrounding world, I felt the urge to look through this window, hoping to see something unexpected.1 BEGINNINGS RUNO ROSSI WAS BORN April 13, 1905, in Venice, the el- Bdest of three sons of Rino Rossi and Lina Minerbi. His father was an electrical engineer whose successful career began with work on the electrification of Venice. He wrote in his autobiography2 that his father loved science and would have chosen it for a career except for practical consider- ations. He attributes to his father the influence that turned what may have been an “inborn tendency toward science . into a lifelong commitment.” He recalled: perfectly clear winter mornings when the air was so unusually transparent that the Alps surrounding Venice became clearly visible and appeared in- credibly close (Fata Morgana if you are a child or a poet, anomalous atmo- spheric refraction if you are a scientist). On those mornings I would try to find a sandalo (a small gondola) and, accompanied by a friend, I would 3 4 BIOGRAPHICAL MEMOIRS row standing on the stern, Venetian style, from the Lido, where I lived, to the school in Venice, where I studied. -

Nobel Prizes Social Network
Nobel prizes social network Marie Skłodowska Curie (Phys.1903, Chem.1911) Nobel prizes social network Henri Becquerel (Phys.1903) Pierre Curie (Phys.1903) = Marie Skłodowska Curie (Phys.1903, Chem.1911) Nobel prizes social network Henri Becquerel (Phys.1903) Pierre Curie (Phys.1903) = Marie Skłodowska Curie (Phys.1903, Chem.1911) Irène Joliot-Curie (Chem.1935) Nobel prizes social network Henri Becquerel (Phys.1903) Pierre Curie (Phys.1903) = Marie Skłodowska Curie (Phys.1903, Chem.1911) Irène Joliot-Curie (Chem.1935) = Frédéric Joliot-Curie (Chem.1935) Nobel prizes social network Henri Becquerel (Phys.1903) Pierre Curie (Phys.1903) = Marie Skłodowska Curie (Phys.1903, Chem.1911) Paul Langevin Irène Joliot-Curie (Chem.1935) = Frédéric Joliot-Curie (Chem.1935) Nobel prizes social network Henri Becquerel (Phys.1903) Pierre Curie (Phys.1903) = Marie Skłodowska Curie (Phys.1903, Chem.1911) Paul Langevin Maurice de Broglie Louis de Broglie (Phys.1929) Irène Joliot-Curie (Chem.1935) = Frédéric Joliot-Curie (Chem.1935) Nobel prizes social network Sir J. J. Thomson (Phys.1906) Henri Becquerel (Phys.1903) Pierre Curie (Phys.1903) = Marie Skłodowska Curie (Phys.1903, Chem.1911) Paul Langevin Maurice de Broglie Louis de Broglie (Phys.1929) Irène Joliot-Curie (Chem.1935) = Frédéric Joliot-Curie (Chem.1935) Nobel prizes social network (more) Sir J. J. Thomson (Phys.1906) Nobel prizes social network (more) Sir J. J. Thomson (Phys.1906) Owen Richardson (Phys.1928) Nobel prizes social network (more) Sir J. J. Thomson (Phys.1906) Owen Richardson (Phys.1928) Clinton Davisson (Phys.1937) Nobel prizes social network (more) Sir J. J. Thomson (Phys.1906) Owen Richardson (Phys.1928) Charlotte Richardson = Clinton Davisson (Phys.1937) Nobel prizes social network (more) Sir J. -

Europe's Biggest General Science Conference Concludes Successfully
SCIENCEScience PagesPAGES Special Report - ESOF 2016 Europe’s biggest generalSpecial Report science conference concludes successfully ESOF 2016, Europe's biggest general science conference concludes successfully in Manchester,in Manchester,UK UK Theme: Science as Revolution Theme: Science as Revolution - Veena Patwardhan rom 23rd to 27th July, 2016, FManchester flaunted its City of Science status as the host city of the seventh edition of EuroScience Open Forum (ESOF 2016). A bi- ennial event held in a different European city every two years, this time it was Manchester's turn to host this globally reputed science conference. Around 4500 delegates – scien- tists, innovators, academics, young researchers, journalists, policy makers, industry representatives and others – converged on the world's first industrial city to dis- cover and have discussions about the latest advancements in scien- rd th Manchester Central, venue of ESOF 2016 tific and technological researchFrom 23 to 27 July, 2016, Manchester flaunted its City of Science status as the host city of the across Europe and beyond. The seventhmain theme edition this of EuroScienceyear Laureates Open Forumand distinguished (ESOF 2016). Ascientists biennial inevent the held packed in a different was 'Science as Revolution', indicatingEuropean that city the every focus two of years,Exchange this time Hall it wasof Manchester Manchester's Central, turn to hostthe venuethis globally of the reputed the conference would be on how sciencescience andconference. technology conference. could transform life on the planet, revolutionise econo- The proceedings began with a string quartet render- mies, and help in overcoming challenges faced by global ing a piece of specially composed music. -

The Nobel Laureate George De Hevesy (1885-1966) - Universal Genius and Father of Nuclear Medicine Niese S* Am Silberblick 9, 01723 Wilsdruff, Germany
Open Access SAJ Biotechnology LETTER ISSN: 2375-6713 The Nobel Laureate George de Hevesy (1885-1966) - Universal Genius and Father of Nuclear Medicine Niese S* Am Silberblick 9, 01723 Wilsdruff, Germany *Corresponding author: Niese S, Am Silberblick 9, 01723 Wilsdruff, Germany, Tel: +49 35209 22849, E-mail: [email protected] Citation: Niese S, The Nobel Laureate George de Hevesy (1885-1966) - Universal Genius and Father of Nuclear Medicine. SAJ Biotechnol 5: 102 Article history: Received: 20 March 2018, Accepted: 29 March 2018, Published: 03 April 2018 Abstract The scientific work of the universal genius the Nobel Laureate George de Hevesy who has discovered and developed news in physics, chemistry, geology, biology and medicine is described. Special attention is given to his work in life science which he had done in the second half of his scientific career and was the base of the development of nuclear medicine. Keywords: George de Hevesy; Radionuclides; Nuclear Medicine Introduction George de Hevesy has founded Radioanalytical Chemistry and Nuclear Medicine, discovered the element hafnium and first separated stable isotopes. He was an inventor in many disciplines and his interest was not only focused on the development and refinement of methods, but also on the structure of matter and its changes: atoms, molecules, cells, organs, plants, animals, men and cosmic objects. He was working under complicated political situation in Europe in the 20th century. During his stay in Germany, Austria, Hungary, Switzerland, Denmark, and Sweden he wrote a lot papers in German. In 1962 he edited a large part of his articles in a collection where German papers are translated in English [1]. -

Marischal College
The Scientific Tourist: Aberdeen Marischal College George P Thomson – Nobel Prize winning physicist Sir George Paget Thomson FRS (1892-1975) was the only son of Sir J. J. Thomson, the Cavendish Professor of Physics who is probably most famous for discovering the existence of the electron and measuring its mass. G. P. Thomson was one of a select group of academics in the University of Aberdeen to be awarded the Nobel Prize. He earned it for his work in the Department of Natural Philosophy at Marischal College during his tenure from 1922-1930. There is now a commemorative plaque in the Marischal College quadrangle. Thomson was a high achieving Trinity College, Cambridge, scholar who graduated in 1913 and was elected Fellow and Lecturer at Corpus Christi College in the following year. He was, though, of the generation whose adult lives were to be shaped by two World Wars and their aftermaths. Thomson spent most of the First World War in France and in the research wing of the Royal Flying Corps. His first book1 came out of this experience and is simply entitled “Applied Aerodynamics”. It is a well-illustrated summary of the lessons of wartime work on the science of aircraft design and operation, underlining the basic physics involved. Upon Thomson’s return to Cambridge he took up research into his father’s long-standing interest of electrical discharges in gases. He brought this interest with him when he came to Aberdeen in 1922 and did much of the work enlarging J. J. Thomson’s “Conduction of Electricity through Gases” into a long awaited 3rd edition that was published in their joint names in 1928. -

ARIE SKLODOWSKA CURIE Opened up the Science of Radioactivity
ARIE SKLODOWSKA CURIE opened up the science of radioactivity. She is best known as the discoverer of the radioactive elements polonium and radium and as the first person to win two Nobel prizes. For scientists and the public, her radium was a key to a basic change in our understanding of matter and energy. Her work not only influenced the development of fundamental science but also ushered in a new era in medical research and treatment. This file contains most of the text of the Web exhibit “Marie Curie and the Science of Radioactivity” at http://www.aip.org/history/curie/contents.htm. You must visit the Web exhibit to explore hyperlinks within the exhibit and to other exhibits. Material in this document is copyright © American Institute of Physics and Naomi Pasachoff and is based on the book Marie Curie and the Science of Radioactivity by Naomi Pasachoff, Oxford University Press, copyright © 1996 by Naomi Pasachoff. Site created 2000, revised May 2005 http://www.aip.org/history/curie/contents.htm Page 1 of 79 Table of Contents Polish Girlhood (1867-1891) 3 Nation and Family 3 The Floating University 6 The Governess 6 The Periodic Table of Elements 10 Dmitri Ivanovich Mendeleev (1834-1907) 10 Elements and Their Properties 10 Classifying the Elements 12 A Student in Paris (1891-1897) 13 Years of Study 13 Love and Marriage 15 Working Wife and Mother 18 Work and Family 20 Pierre Curie (1859-1906) 21 Radioactivity: The Unstable Nucleus and its Uses 23 Uses of Radioactivity 25 Radium and Radioactivity 26 On a New, Strongly Radio-active Substance