Hawthorn (Crataegus Laevigata, C. Oxyacantha, C. Monogyna, C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Midlan Hawthorn
18 F L E S H Y F R U I T S F L E S H Y F R U I T S 19 Hawthorn - Crataegus monogyna Midland Hawthorn - Crataegus laevigata idland hawthorn is the he native hawthorn grows throughout Mrarer of the two native TBritain, except in the extreme north-west hawthorn species and the of Scotland. It tolerates most soils except peat one that is more likely to and is probably best known as a plant of grow into a small tree. It is hedgerows. found on heavy soils in shady The wood has been used for tool handles woodlands, or growing along road- and walking sticks and also produces excellent sides and in gardens, usually in the firewood and charcoal. The berries can be south east of Britain. Many ornamen- made into jellies, chutneys and wine. tal forms have been created, including The species has a high wildlife value, ‘Paul’s Scarlet’, which is frequently seen as its flowers provide nectar for spring insects in towns and cities. and its berries provide excellent food for small Medium tree (6:8:10) As with hawthorn, the wood has been mammals and birds, especially thrushes. Over used for tool handles and walking sticks, 209 invertebrate species have been recorded its fluted stems making it particularly use- living on this tree. ful for the latter. The berries can be made into jellies, chutneys and wine. Seed Guide: Collect the red berries, once they are ripe, from autumn onwards. Remove the The leaves have deeply divided The flowers provide nectar for spring lobes.The fleshy red berries (haws) insects, the berries excellent food for seeds from the flesh and wash them thorough- contain a single seed. -

ECOLOGICAL and ECONOMIC IMPORTANCE of STUDYING PROPAGATION TECHNIQUES of COMMON HAWTHORN Crataegus Monogyna Jacq. G
СИБИРСКИЙ ЛЕСНОЙ ЖУРНАЛ. 2019. № 4. С. 63–67 UDC 581.16/581.6/581.9:634.17 ECOLOGICAL AND ECONOMIC IMPORTANCE OF STUDYING PROPAGATION TECHNIQUES OF COMMON HAWTHORN Crataegus monogyna Jacq. G. Özyurt, Z. Yücesan, N. Ak, E. Oktan, A. Ö. Üçler Karadeniz Technical University Trabzon, 61080 Turkey E-mail: [email protected], [email protected], [email protected], [email protected], [email protected] Received 11.04.2019 Climate change as a fact of global warming requires the development of different perspectives on the planning and implementation of sustainable forestry techniques. Increasing temperatures cause drought on a global basis. In connection with, this using drought tolerant species in afforestation work is of great importance. In recent years Crataegus L. species (hawthorn) are also involved in afforestation. One of these species, C. monogyna, is characterized by drought tolerance. Furthermore, C. monogyna is the most important nonwood forest product species of Turkey. Hawthorn is widely used in medicine (treatment of coronary heart diseases), and cosmetics industry, agriculture and animal husbandry and human nutrition. On the other hand, it is used in erosion control, afforestation, industrial energy resources and for landscaping. Economic and ecological contribution of hawthorn to the national economy is quite high. Therefore, determination of suitable generative and vegetative reproduction techniques and vast production of seedlings of hawthorn species are extremely important. The characteristics of generative and vegetative propagation of Crataegus are discussed. For generative propagation of hawthorn species, the most effective and suitable procedure is treatment of seeds in ash solution. For vegetative propagation in culture in vitro the growth induced by BA (benzyladenine) and IBA (indole butyric acid) hormones increases the rate of callus formation and rooting. -
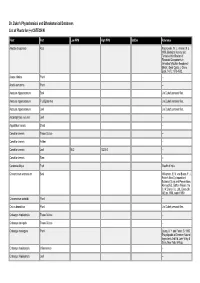
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN Plant Part Low PPM High PPM StdDev Reference Abutilon theophrasti Root Paszkowski, W. L., Kremer, R. J. 1988. Biological Activity and Tentative Identification of Flavonoid Components in Velvetleaf (Abutilon theophrasti Medik.) Seed Coats. J. Chem. Ecol., 14(7): 1573-1582. Acacia nilotica Plant -- Acacia decurrens Plant -- Aesculus hippocastanum Bark Jim Duke's personal files. Aesculus hippocastanum Fruit Epidermis Jim Duke's personal files. Aesculus hippocastanum Leaf Jim Duke's personal files. Arctostaphylos uva-ursi Leaf -- Aspalathus linearis Shoot -- Camellia sinensis Tissue Culture -- Camellia sinensis Anther -- Camellia sinensis Leaf 85.2 13200.0 -- Camellia sinensis Stem -- Ceratonia siliqua Fruit Wealth of India. Cinnamomum aromaticum Bark Williamson, E. M. and Evans, F. J., Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed., Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 362 pp, 1988, reprint 1989. Cinnamomum sieboldii Plant -- Cnicus benedictus Plant Jim Duke's personal files. Crataegus rhipidophylla Tissue Culture -- Crataegus laevigata Tissue Culture -- Crataegus monogyna Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Inflorescence -- Crataegus rhipidophylla Leaf -- Plant Part Low PPM High PPM StdDev Reference Crataegus laevigata Leaf -- Crataegus laevigata Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Bud -- Crataegus rhipidophylla Flower -- Crataegus laevigata Bud -- Crataegus laevigata Flower -- Croton lechleri Plant -- Eucalyptus globulus Leaf -- Fagopyrum esculentum Root -- Fagopyrum esculentum Tissue Culture -- Fallopia japonica Root 2.0 -- Geranium thunbergii Tissue Culture -- Ginkgo biloba Tissue Culture Jim Duke's personal files. -

Cambridgeshire and Peterborough County Wildlife Sites
Cambridgeshire and Peterborough County Wildlife Sites Selection Guidelines VERSION 6.2 April 2014 CAMBRIDGESHIRE & PETERBOROUGH COUNTY WILDLIFE SITES PANEL CAMBRIDGESHIRE & PETERBOROUGH COUNTY WILDLIFE SITES PANEL operates under the umbrella of the Cambridgeshire and Peterborough Biodiversity Partnership. The panel includes suitably qualified and experienced representatives from The Wildlife Trust for Bedfordshire, Cambridgeshire, Northamptonshire; Natural England; The Environment Agency; Cambridgeshire County Council; Peterborough City Council; South Cambridgeshire District Council; Huntingdonshire District Council; East Cambridgeshire District Council; Fenland District Council; Cambridgeshire and Peterborough Environmental Records Centre and many amateur recorders and recording groups. Its aim is to agree the basis for site selection, reviewing and amending them as necessary based on the best available biological information concerning the county. © THE WILDLIFE TRUST FOR BEDFORDSHIRE, CAMBRIDGESHIRE AND NORTHAMPTONSHIRE 2014 © Appendices remain the copyright of their respective originators. All rights reserved. Without limiting the rights under copyright reserved above, no part of this publication may be reproduced, stored in any type of retrieval system or transmitted in any form or by any means (electronic, photocopying, mechanical, recording or otherwise) without the permission of the copyright owner. INTRODUCTION The Selection Criteria are substantially based on Guidelines for selection of biological SSSIs published by the Nature Conservancy Council (succeeded by English Nature) in 1989. Appropriate modifications have been made to accommodate the aim of selecting a lower tier of sites, i.e. those sites of county and regional rather than national importance. The initial draft has been altered to reflect the views of the numerous authorities consulted during the preparation of the Criteria and to incorporate the increased knowledge of the County's habitat resource gained by the Phase 1 Habitat Survey (1992-97) and other survey work in the past decade. -

Crataegus Laevigata 'Crimson Cloud' 'Crimson Cloud' English Hawthorn
Fact Sheet ST-211 November 1993 Crataegus laevigata ‘Crimson Cloud’ ‘Crimson Cloud’ English Hawthorn1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Crimson Cloud (also known as ‘Superba’) English Hawthorn grows rapidly in a pyramidal form to about 20 feet, then the crown expands to become oval or irregular (Fig. 1). The tree tolerates most soils, growing well in clay, but prefers heavy, dry loam. The main ornamental feature is white and red flowers borne in spring which together give the tree a deep pink color. Fruits are red and quite showy but do not cover the tree. Though quite ornamental, Hawthorns are susceptible to insect and disease problems. Branching habit is decidedly drooping and care should be given when locating this tree near pedestrian or vehicular traffic. GENERAL INFORMATION Figure 1. Middle-aged ‘Crimson Cloud’ English Hawthorn. Scientific name: Crataegus laevigata ‘Crimson Cloud’ Availability: grown in small quantities by a small Pronunciation: kruh-TEE-gus lee-vih-GAY-tuh number of nurseries Common name(s): ‘Crimson Cloud’ English Hawthorn DESCRIPTION Family: Rosaceae USDA hardiness zones: 4B through 8 (Fig. 2) Height: 20 to 25 feet Origin: not native to North America Spread: 15 to 25 feet Uses: Bonsai; espalier; wide tree lawns (>6 feet Crown uniformity: irregular outline or silhouette wide); medium-sized tree lawns (4-6 feet wide); Crown shape: oval; pyramidal recommended for buffer strips around parking lots or Crown density: moderate for median strip plantings in the highway; reclamation Growth rate: medium plant; screen; narrow tree lawns (3-4 feet wide); Texture: fine specimen; sidewalk cutout (tree pit); residential street tree; tree has been successfully grown in urban areas where air pollution, poor drainage, compacted soil, and/or drought are common 1. -

(Rosaceae), I. Differentiation of Mespilus and Crataegus
Phytotaxa 257 (3): 201–229 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2016 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.257.3.1 STUDIES IN MESPILUS, CRATAEGUS, AND ×CRATAEMESPILUS (ROSACEAE), I. DIFFERENTIATION OF MESPILUS AND CRATAEGUS, EXPANSION OF ×CRATAEMESPILUS, WITH SUPPLEMENTARY OBSERVATIONS ON DIFFERENCES BETWEEN THE CRATAEGUS AND AMELANCHIER CLADES JAMES B. PHIPPS Department of Biology, The University of Western Ontario, London, Ontario N6A 5B7, Canada; email: [email protected] Abstract The paper argues the position for retaining a monotypic Mespilus, i.e., in the sense of M. germanica, the medlar. Recent cladistic papers lend support for Mespilus being sister to Crataegus, and there is a clear morphological distinction from Cra- taegus, emphasized by adaptation to carnivore frugivory. Mespilus secured, the paper then treats each of the known hybrids between Mespilus and Crataegus, making the new combination Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps. Keywords: Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps comb. nov.; inflorescence position; medlar; Mespilus a folk-genus; Mespilus distinct from Crataegus; Rosaceae; taxonomic history of Mespilus Introduction The author has a long-standing interest in generic delimitation in the Maloid genera of the Rosaceae (Maleae Small, formerly Maloideae C. Weber, Pyrinae Dumort.), as shown particularly in a series of papers with K. Robertson, J. Rohrer, and P.G. Smith (Phipps et al. 1990, 1991; Robertson at al. 1991, 1992; Rohrer at al. 1991, 1994) which treated all 28 genera of Maleae as recognised by us. There is also a revisionary treatment of New World Heteromeles M.J. -

Crataegus ×Media 'Paul's Scarlet'
All the knowledge. Almost all of the trees. https://www.vdberk.co.uk/trees/crataegus-media-paul-s-scarlet/ Crataegus ×media 'Paul's Scarlet' Height 6 - 8 (10) m Crown broad spherical, later rounded, half-open crown, capricious growing Bark and branches bark brownish-grey, flaking off in platelets, twigs thorny Leaf ovoid, 3/5-lobed, dark green, 3 - 6 cm Flowers red double flowers in umbels, May-June Fruits none, fruitless cultivar Spines/thorns Yes Toxicity usually not toxic to people, (large) pets and livestock Soil type makes few demands, preferably not too dry Paving tolerates paving Winter hardiness zone 5a (-28,8 to -26,1 °C) Wind resistance moderate Other resistances resistant to frost (WH 1 - 6) Fauna tree resistant to frost (WH 1 - 6), valuable for bees (honey plant) Application avenues and broad streets, parks, squares, cemeteries, large gardens, small gardens Shape clearstem tree, multi-stem treem Origin Wm. Paul, England, 1860 Synonyms Crataegus laevigata 'Paul's Scarlet' Sometimes grown as a shrub but mostly sold as a tree. The sturdy lateral branches that grow outwards create a more or less rounded open type of crown. It can eventually attain a maximum width of 6 to 7 m. The trunk and branches are grey. The branches are less thorny than those of the species. The thorns are approx. 2.5 cm long and viciously sharp. The dark green leaves are lighter on the underside. The entire tree is covered with blossom in the spring. The flowers turn from even to pale red. The tough hard roots go deep and spread wide. -

Trees to Avoid Planting in the Midwest and Some Excellent Alternatives
Trees to Avoid Planting in the Midwest and Some Excellent Alternatives Dr. Laura G. Jull Dept. of Horticulture, UW-Madison Trees provide us with many environmental, aesthetic, functional, and economic benefits. Tree selection is one of the most important considerations when a homeowner, nurserymen, or landscaper is deciding what species to grow or plant. Many questions need to be answered including size, location, site characteristics, aesthetic features, pest susceptibility, hardiness, and maintenance considerations. Some trees can become a maintenance headache due to their inherent pest problems or lack of structural integrity. The trees represented in this story have not generally performed well in urban and suburban areas of the Midwest. Some are susceptible to insects and diseases, and some have severe structural problems such as being weak- wooded or prone to girdling roots or included bark formation. Others have cultural problems such as intolerance to high pH, road salt, drought, and poor drainage. A few tree species are invasive and should be avoided near sensitive areas or seed dispersal into woodlands could occur. Some of these trees may do quite well in other parts of the U.S., so my intention is not to apply a blanket statement for all these trees to all situations. Invasiveness and pest susceptibility can vary geographically. The article is based on more than 25 years of field experience and data collected from numerous states’ plant disease and insect diagnostic clinics, and conversations with arborists, nurseries, landscapers, and extension personnel. There are alternative species that can be used and are mentioned here. These alternative tree species have performed well in USDA Cold Hardiness Zone 4b. -

3.2 Conservation Value of Scrub
••••••. a a a a a= 11111. a a aaaalaaaa JNCC Report No 308 The nature conservation value of scrub in Britain SR Mortimer.. AJ Turner' VK Brown', RJ Fuller'. JEG Goods SA Bell'. PA Stevens'. D Norris', N Bayfieldn, & LK Ward' August 2000 This report should be cited as: Mortimer. SR. Turner. Al. Brown, VIC,Fuller, RJ, Good. JEG, Bell, SA. Stevens. PA. Norris. D. Bayfield. N & Ward, LK 2000. TI The nature conservation value of scrub in Britain. JNCC Report No. 308. JNCC. Peterborough 2000 For further information please contact: Habitats Advice Joint Nature Conservation Committee Monkstone House. City Road. Peterborough PEI HY. UK ISSN 0963-8091 CYNCOI cm' CWLAD SCOTTISH CYMRU N=77-",\! NATURAL COUNMSIDI HERITAGE COUNCII Mt WU It ENGLISH NATURE 0-4^70, This report was produced as a result of a commission research contract for English Nature with contributions from Scottish Nature Heritage and the Countryside Council for Wales CABI Bioseienee, Sik%ilod Park. A.eoi. Berks. SI.5 7TA 1- British Trust I-or Ornitholouy. The Nunnery. Thcilord. :Sorkin:. IP24 2PU Centre lor EcoioL:y and Hydoilou . Demo! 12ikid. Bangor. Gviy nedd. LL.57 2U1' II Centre tor licidoey and Ilydroloy. I lill uI Brathens. Glasse!. Banchory. Kincardineshire AB3 I 413Y + 53 Nide, Avenue. Sandtord. Wareham. Dorset. 131120 7AS 1 JOINT NATURE CONSERVATION COMMITTEE: REPORT DISTRIBUTION Report number 308 Report title: The nature conservation value of scrub Contract number: FIN/CON/VT998 Nominated Officer Jeanette Hall. Woodland Network Liaison Officer Date received: April 20110 Contract title: A review of the nature conservation value of scrub in the UK Contractors: CABI Bioscience. -

The Timeless Beneficial Bloom Herb Profile Hawthorn Crataegus Monogyna, C
HerbalGram 96 • November 2012 - January 2013 HerbalGram 96 • November 2012 - January 2013 Largest Echinacea Trial • Bilberry Adulteration • Sports and Supplements Federal Cannabis Crackdown • Bacopa and Memory • Butterbur and Migraine The Journal of the American Botanical Council Number 96 | November 2012 - January 2013 Turkish RoseTurkish • Trial Largest • Echinacea Bilberry Adulteration • Sports and Supplements • Cannabis Crackdown Federal • Bacopa and Memory • Butterbur and Migraine US/CAN $6.95 www.herbalgram.org Rose www.herbalgram.org The Timeless Beneficial Bloom Herb Profile Hawthorn Crataegus monogyna, C. laevigata Family: Rosaceae INTRODUCTION HISTORY AND CULTURAL SIGNIFICANCE Hawthorn is a large shrub or small tree (15-30 feet on The generic name, Crataegus, comes from the Greek kratos, average) in the genus Crataegus, native to temperate North meaning hard or strong, referring to the plant’s wood.13,14 America, Europe, and East Asia.1 The plants are indetermi- The common name refers to the plant’s thorns and fruit, nately thorny, with variable shape, and have perfect, radially known as haws, and may also refer to its use to form hedges, symmetrical, 5-petaled white to pink flowers (red in some which were called haws in earlier times.13 Other common M I S S I O N D R I V E N : cultivars) in corymbs (flat-topped clusters).2,3 The red fruit names for C. laevigata include English hawthorn, white are drupes (one-seeded and fleshy) but are commonly called thorn, May tree (referring to when it blooms), and two- berries in the trade. The genus Crataegus comprises approxi- style hawthorn; and English hawthorn, one-seed hawthorn, mately 250-280 species, the most commonly used in West- and one-style hawthorn for C. -

The Plant List
the list A Companion to the Choosing the Right Plants Natural Lawn & Garden Guide a better way to beautiful www.savingwater.org Waterwise garden by Stacie Crooks Discover a better way to beautiful! his plant list is a new companion to Choosing the The list on the following pages contains just some of the Right Plants, one of the Natural Lawn & Garden many plants that can be happy here in the temperate Pacific T Guides produced by the Saving Water Partnership Northwest, organized by several key themes. A number of (see the back panel to request your free copy). These guides these plants are Great Plant Picks ( ) selections, chosen will help you garden in balance with nature, so you can enjoy because they are vigorous and easy to grow in Northwest a beautiful yard that’s healthy, easy to maintain and good for gardens, while offering reasonable resistance to pests and the environment. diseases, as well as other attributes. (For details about the GPP program and to find additional reference materials, When choosing plants, we often think about factors refer to Resources & Credits on page 12.) like size, shape, foliage and flower color. But the most important consideration should be whether a site provides Remember, this plant list is just a starting point. The more the conditions a specific plant needs to thrive. Soil type, information you have about your garden’s conditions and drainage, sun and shade—all affect a plant’s health and, as a particular plant’s needs before you purchase a plant, the a result, its appearance and maintenance needs. -

Whole Plant Based Treatment of Hypercholesterolemia with Crataegus Laevigata in a Zebrafish Model Robert M Littleton1, Matthew Miller1,2 and Jay R Hove1,2*
Littleton et al. BMC Complementary and Alternative Medicine 2012, 12:105 http://www.biomedcentral.com/1472-6882/12/105 RESEARCH ARTICLE Open Access Whole plant based treatment of hypercholesterolemia with Crataegus laevigata in a zebrafish model Robert M Littleton1, Matthew Miller1,2 and Jay R Hove1,2* Abstract Background: Consumers are increasingly turning to plant-based complementary and alternative medicines to treat hypercholesterolemia. Many of these treatments are untested and their efficacy is unknown. This multitude of potential remedies necessitates a model system amenable to testing large numbers of organisms that maintains similarity to humans in both mode of drug administration and overall physiology. Here we develop the larval zebrafish (4–30 days post fertilization) as a vertebrate model of dietary plant-based treatment of hypercholesterolemia and test the effects of Crataegus laevigata in this model. Methods: Larval zebrafish were fed high cholesterol diets infused with fluorescent sterols and phytomedicines. Plants were ground with mortar and pestle into a fine powder before addition to food. Fluorescent sterols were utilized to optically quantify relative difference in intravascular cholesterol levels between groups of fish. We utilized the Zeiss 7-Live Duo high-speed confocal platform in order to both quantify intravascular sterol fluorescence and to capture video of the heart beat for determination of cardiac output. Results: In this investigation we developed and utilized a larval zebrafish model to investigate dietary plant-based intervention of the pathophysiology of hypercholesterolemia. We found BODIPY-cholesterol effectively labels diet- introduced intravascular cholesterol levels (P < 0.05, Student’s t-test). We also established that zebrafish cardiac output declines as cholesterol dose increases (difference between 0.1% and 8% (w/w) high cholesterol diet-treated cardiac output significant at P < 0.05, 1-way ANOVA).