Crataegus (Hawthorn)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cockspur Hawthorn Grows from Southern Quebec, and Ontario to Northern Louisiana, Alabama and Northwestern Georgia, and West to Kansas
Plant Guide COCKSPUR Status Please consult the PLANTS Web site and your State HAWTHORN Department of Natural Resources for this plant’s current status, such as, state noxious status, and Crataegus crus-galli L. wetland indicator values. Plant Symbol = CRCR2 Description Contributed By: USDA NRCS National Plant Data General: It is a small tree that grows twenty to thirty Center feet high, twenty to thirty-five feet wide; with wide- spreading, horizontal, thorny branches. Leaves are broadest above or near the middle, thick, leathery, shiny dark green above, usually not lobed, and smooth. Flowers ranging from white to red are produced in clusters. Fruits are broadest above the middle or rounded, dull red or green. Distribution: Cockspur hawthorn grows from southern Quebec, and Ontario to northern Louisiana, Alabama and northwestern Georgia, and west to Kansas. Adaptation Although Cockspur hawthorn generally requires no special soil requirements, it prefers a moist, well- drained, slightly acid soils, and full sunlight. It is adaptable to poor soils; various soil pHs, compacted soils, drought, heat and winter salt spray. Adapted to USDA Hardiness Zone 4. Establishment Propagation from Seed or Grafting: Cockspur hawthorn can be propagated by seeds or by stem Native Trees of Texas Department of Horticulture cuttings grafted onto seedling rootstock. Propagation Texas A&M University using seeds requires acid scarification for two to Uses three hours followed by three months warm Erosion Control: Because it tolerates a wide variety stratification and another three months prechilling. of sites, it can be planted to stabilize banks, for Seeds are planted in drill rows eight to twelve inches shelterbelts, and for erosion control. -

Shrub List for Brighton 2010
Shrub List For Brighton 2010 Large Shrubs 10’ -20’ Tall by 6’ – 25’ wide Acer ginnala Amur Maple Acer tataricum Tatarian Maple (better than Amur Maple) Acer grandidentatum Bigtooth Maple Amelanchier alnifolia Saskatoon Serviceberry Amelanchier canadensis Shadblow Serviceberry Caragana arborescens Siberian Peashrub Cercocarpus ledifolius Mountain Mahogany Cotoneaster lucidus Peking Cotoneaster Cowania mexicana Quince Bush, Cliffrose Crataefus ambigua Russian Hawthorn Forestiera neomexicana New Mexican Privet Hippophae rhamnoides Sea Buckthorn Juniperus species Juniper Kolkwitzia amabilis Beauty Bush Pinus mugo Mugo Pine species Prunus americana American Plum Prunus virginiana ‘Shubert’ Canada Red Chokecherry Ptelea trifoliata Wafer Ash or Hop tree Quercus gambelii Gambel Oak Rhus typhina Staghorn Sumac Robinia neomexicana New Mexico Locust Sambucus species Elders Shepherdia argentea Buffaloberry Syringa vulgaris Common Lilac Viburnum lantana Wayfaring Tree, Viburnum Medium Size Shrubs >10’ high by >8’ wide Amorpha fruticosa False Indigo Atriplex canescens Fourwing Saltbush Buddleia davidii Butterfly Bush Cercocarpus montanus Mountain Mahogany Chamaebatiaria millefolium Fernbush Chrysothamnus nauseosus Rubber Rabbitbrush Cornus sericea Redtwig Dogwood Cotinus coggygria Smoke Tree Cotoneaster species Cotoneaster Cytisus scoparius ‘Moonlight’ Moonlight Broom Euonymus alatus Burning Bush Forsythia x intermedia Forsythia Hibiscus syriacus Rose-of-Sharon Juniperus species Juniper Ligustrum vulgare Privet Lonicera species Honeysuckle Mahonia aquifolium Oregon Grape Holly Philadelphus species Mockorange Pyracantha coccinea Firethorn Physocarpus opulifolius Common Ninebark Prunus besseyi Western Sand Cherry Pyracantha coccinea species Firethorn Rhamnus frangula Glossy Buckthorn Ribes species Currant Sambucus species Elder Spiraea x vanhouttei Vanhouttei Spirea Symphoricarpos albus Snowberry Syringa meyeri „Palibin‟ Dwarf Korean Lilac Syringa patula „Miss Kim‟ Dwarf Lilac Viburnum species (dozens of different types) Small Size Shrubs > 5’ tall by >6. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Midlan Hawthorn
18 F L E S H Y F R U I T S F L E S H Y F R U I T S 19 Hawthorn - Crataegus monogyna Midland Hawthorn - Crataegus laevigata idland hawthorn is the he native hawthorn grows throughout Mrarer of the two native TBritain, except in the extreme north-west hawthorn species and the of Scotland. It tolerates most soils except peat one that is more likely to and is probably best known as a plant of grow into a small tree. It is hedgerows. found on heavy soils in shady The wood has been used for tool handles woodlands, or growing along road- and walking sticks and also produces excellent sides and in gardens, usually in the firewood and charcoal. The berries can be south east of Britain. Many ornamen- made into jellies, chutneys and wine. tal forms have been created, including The species has a high wildlife value, ‘Paul’s Scarlet’, which is frequently seen as its flowers provide nectar for spring insects in towns and cities. and its berries provide excellent food for small Medium tree (6:8:10) As with hawthorn, the wood has been mammals and birds, especially thrushes. Over used for tool handles and walking sticks, 209 invertebrate species have been recorded its fluted stems making it particularly use- living on this tree. ful for the latter. The berries can be made into jellies, chutneys and wine. Seed Guide: Collect the red berries, once they are ripe, from autumn onwards. Remove the The leaves have deeply divided The flowers provide nectar for spring lobes.The fleshy red berries (haws) insects, the berries excellent food for seeds from the flesh and wash them thorough- contain a single seed. -

Scientific Name Species Common Name Abies Lasiocarpa FIR Subalpine Acacia Macracantha ACACIA Long-Spine
Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek Alnus iridis ssp. sinuata ALDER Sitka Alnus maritima ALDER Seaside Alvaradoa amorphoides ALVARADOA Mexican Amelanchier laevis SERVICEBERRY Allegheny Amyris balsamifera TORCHWOOD Balsam Annona squamosa SUGAR-APPLE NA Araucaria cunninghamii ARAUCARIA Cunningham Arctostaphylos glauca MANZANITA Bigberry Asimina obovata PAWPAW Bigflower Bourreria radula STRONGBACK Rough Brasiliopuntia brasiliensis PRICKLY-PEAR Brazilian Bursera simaruba GUMBO-LIMBO NA Caesalpinia pulcherrima FLOWERFENCE NA Capparis flexuosa CAPERTREE Limber CRUCIFIXION- Castela emoryi THORN NA Casuarina equisetifolia CASUARINA Horsetail Ceanothus arboreus CEANOTHUS Feltleaf Ceanothus spinosus CEANOTHUS Greenbark Celtis lindheimeri HACKBERRY Lindheimer Celtis occidentalis HACKBERRY Common Cephalanthus occidentalis BUTTONBUSH Common Cercis canadensis REDBUD Eastern Cercocarpus traskiae CERCOCARPUS Catalina Chrysophyllum oliviforme SATINLEAF NA Citharexylum berlandieri FIDDLEWOOD Berlandier Citrus aurantifolia LIME NA Citrus sinensis ORANGE Orange Coccoloba uvifera SEAGRAPE NA Colubrina arborescens COLUBRINA Coffee Colubrina cubensis COLUBRINA Cuba Condalia globosa -

Amelanchierspp. Family: Rosaceae Serviceberry
Amelanchier spp. Family: Rosaceae Serviceberry The genus Amelanchier contains about 16 species native to North America [5], Mexico [2], and Eurasia to northern Africa [4]. The word amelanchier is derived from the French common name amelanche of the European serviceberry, Amelanchier ovalis. Amelanchier alnifolia-juneberry, Pacific serviceberry, pigeonberry, rocky mountain servicetree, sarvice, sarviceberry, saskatoon, saskatoon serviceberry, western service, western serviceberry , western shadbush Amelanchier arborea-Allegheny serviceberry, apple shadbush, downy serviceberry , northern smooth shadbush, shadblow, shadblown serviceberry, shadbush, shadbush serviceberry Amelanchier bartramiana-Bartram serviceberry Amelanchier canadensis-American lancewood, currant-tree, downy serviceberry, Indian cherry, Indian pear, Indian wild pear, juice plum, juneberry, may cherry, sugar plum, sarvice, servicetree, shadberry, shadblow, shadbush, shadbush serviceberry, shadflower, thicket serviceberry Amelanchier florida-Pacific serviceberry Amelanchier interior-inland serviceberry Amelanchier sanguinea-Huron serviceberry, roundleaf juneberry, roundleaf serviceberry , shore shadbush Amelanchier utahensis-Utah serviceberry Distribution In North America throughout upper elevations and temperate forests. The Tree Serviceberry is a shrub or tree that reaches a height of 40 ft (12 m) and a diameter of 2 ft (0.6 m). It grows in many soil types and occurs from swamps to mountainous hillsides. It flowers in early spring, producing delicate white flowers, making -

ECOLOGICAL and ECONOMIC IMPORTANCE of STUDYING PROPAGATION TECHNIQUES of COMMON HAWTHORN Crataegus Monogyna Jacq. G
СИБИРСКИЙ ЛЕСНОЙ ЖУРНАЛ. 2019. № 4. С. 63–67 UDC 581.16/581.6/581.9:634.17 ECOLOGICAL AND ECONOMIC IMPORTANCE OF STUDYING PROPAGATION TECHNIQUES OF COMMON HAWTHORN Crataegus monogyna Jacq. G. Özyurt, Z. Yücesan, N. Ak, E. Oktan, A. Ö. Üçler Karadeniz Technical University Trabzon, 61080 Turkey E-mail: [email protected], [email protected], [email protected], [email protected], [email protected] Received 11.04.2019 Climate change as a fact of global warming requires the development of different perspectives on the planning and implementation of sustainable forestry techniques. Increasing temperatures cause drought on a global basis. In connection with, this using drought tolerant species in afforestation work is of great importance. In recent years Crataegus L. species (hawthorn) are also involved in afforestation. One of these species, C. monogyna, is characterized by drought tolerance. Furthermore, C. monogyna is the most important nonwood forest product species of Turkey. Hawthorn is widely used in medicine (treatment of coronary heart diseases), and cosmetics industry, agriculture and animal husbandry and human nutrition. On the other hand, it is used in erosion control, afforestation, industrial energy resources and for landscaping. Economic and ecological contribution of hawthorn to the national economy is quite high. Therefore, determination of suitable generative and vegetative reproduction techniques and vast production of seedlings of hawthorn species are extremely important. The characteristics of generative and vegetative propagation of Crataegus are discussed. For generative propagation of hawthorn species, the most effective and suitable procedure is treatment of seeds in ash solution. For vegetative propagation in culture in vitro the growth induced by BA (benzyladenine) and IBA (indole butyric acid) hormones increases the rate of callus formation and rooting. -

The Typification of Cotoneaster Symondsii (Rosaceae)
Phytotaxa 164 (2): 149–153 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2014 Magnolia Press ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.164.2.9 The typification of Cotoneaster symondsii (Rosaceae) JEANETTE FRYER1 & PETER F. ZIKA2,* 1 Cornhill Cottage, Honeycritch Lane, Froxfield, Petersfield, Hampshire, GU32 1BE, England. 2 WTU Herbarium, Box 355325, University of Washington, Seattle, WA 98195-5325, USA. * Author for Correspondence, Email: [email protected] Abstract The binomial Cotoneaster symondsii was published eight years earlier than Cotoneaster simonsii. Some authors have argued that Cotoneaster simonsii should be synonymized under Cotoneaster symondsii, based on priority. Foliar characters provided in the protologue of Cotoneaster symondsii are not a good match for Cotoneaster simonsii. In the absence of original material, a neotype is chosen for Cotoneaster symondsii, in accordance with its protologue, which places it in synonymy with Cotoneaster marginatus. Key words: Cotoneaster subgenus Chaenopetalum, Himalaya, India, nomenclature The binomial Cotoneaster symondsii Moore (1861: 298) was validly published but the original material that formed the basis of the description remains unfound. We were unable to locate any herbarium specimens collected by or seen by Moore when describing C. symondsii. The British Museum received Moore's types and most of his collections that were not ferns (Stafleu & Cowan 1981). We have searched AK, B, BM, CAM, DBN, E, K, KIEV, HILL, OXF, P, WAG, and a number of additional herbaria, but unsuccessfully, and did not find any collections from circa 1860 labeled Cotoneaster symondsii. Moore's (1861) protologue reads: "from Mr. Standish, Bagshot. -

Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Original Research Article
1 Original Research Article 2 3 THE MALOIDEAE (ROSACEAE) STRUCTURAL AND FUNCTIONAL FEATURES 4 DETERMINING PASSIVE IMMUNITY TO MYCOSIS 5 6 7 With the help of microscopic methods the leaves and fruits surface tissues of plants of four 8 genera of the Maloideae subfamily were screened: Malus Mill., Pyrus L., Cydonia Mill., 9 Mespilus L., as model objects, and attempts were made to explain the dependence of mycosis 10 damage on microstructural features. The species composition of fungi that cause damage to the 11 Maloideae leaves and fruits in the Russia southern regions is analyzed. It is established that 12 among pathogens with different types of parasitism there are common excitants, as well as 13 highly specialized, more represented on Mespilus germanica. Higher resistance to the complex 14 of fungal diseases, in comparison with apple and pear, was found in quince and medlar. This 15 stability at the initial stage of the pathological process is associated with structural features such 16 as micromorphology of the fruits and stomata cuticle in the abaxial leaves epidermis. The leaves 17 stomatal cracks of the medlar are narrow with raised outgrowths, on the surface of the fruit – the 18 layered structure of the cuticular layer. Quince has a powerful continuous cuticular cover. 19 Compared with Malus and Pyrus, Cydonia and Mespilus also have a large (30 % or more) 20 polyphenol content in the pericarp outer layer cells. In addition to the gender-specific differences 21 in the microstructure of the integumentary tissues and the content of polyphenols affecting the 22 resistance to pathogens at the stage of their penetration, general patterns of leaf surface 23 formation, such as hypostomacy, anomocytic stomata, folded microrelief of the cuticular surface, 24 and the presence of single and multicellular trichomes are noted. -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -
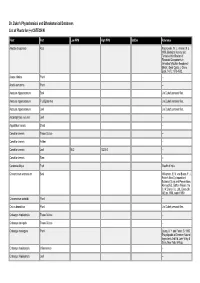
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN Plant Part Low PPM High PPM StdDev Reference Abutilon theophrasti Root Paszkowski, W. L., Kremer, R. J. 1988. Biological Activity and Tentative Identification of Flavonoid Components in Velvetleaf (Abutilon theophrasti Medik.) Seed Coats. J. Chem. Ecol., 14(7): 1573-1582. Acacia nilotica Plant -- Acacia decurrens Plant -- Aesculus hippocastanum Bark Jim Duke's personal files. Aesculus hippocastanum Fruit Epidermis Jim Duke's personal files. Aesculus hippocastanum Leaf Jim Duke's personal files. Arctostaphylos uva-ursi Leaf -- Aspalathus linearis Shoot -- Camellia sinensis Tissue Culture -- Camellia sinensis Anther -- Camellia sinensis Leaf 85.2 13200.0 -- Camellia sinensis Stem -- Ceratonia siliqua Fruit Wealth of India. Cinnamomum aromaticum Bark Williamson, E. M. and Evans, F. J., Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed., Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 362 pp, 1988, reprint 1989. Cinnamomum sieboldii Plant -- Cnicus benedictus Plant Jim Duke's personal files. Crataegus rhipidophylla Tissue Culture -- Crataegus laevigata Tissue Culture -- Crataegus monogyna Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Inflorescence -- Crataegus rhipidophylla Leaf -- Plant Part Low PPM High PPM StdDev Reference Crataegus laevigata Leaf -- Crataegus laevigata Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Bud -- Crataegus rhipidophylla Flower -- Crataegus laevigata Bud -- Crataegus laevigata Flower -- Croton lechleri Plant -- Eucalyptus globulus Leaf -- Fagopyrum esculentum Root -- Fagopyrum esculentum Tissue Culture -- Fallopia japonica Root 2.0 -- Geranium thunbergii Tissue Culture -- Ginkgo biloba Tissue Culture Jim Duke's personal files.