(Rosaceae), I. Differentiation of Mespilus and Crataegus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stomata Size in Relation to Ploidy Level in North American Hawthorns (Crataegus, Rosaceae) Author(S): Brechann V
Stomata Size in Relation to Ploidy Level in North American Hawthorns (Crataegus, Rosaceae) Author(s): Brechann V. McGoey Kelvin Chau Timothy A. Dickinson Source: Madroño, 61(2):177-193. 2014. Published By: California Botanical Society DOI: http://dx.doi.org/10.3120/0024-9637-61.2.177 URL: http://www.bioone.org/doi/full/10.3120/0024-9637-61.2.177 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. MADRON˜ O, Vol. 61, No. 2, pp. 177–193, 2014 STOMATA SIZE IN RELATION TO PLOIDY LEVEL IN NORTH AMERICAN HAWTHORNS (CRATAEGUS,ROSACEAE) BRECHANN V. MCGOEY Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, ON, Canada M5S 3B2 [email protected] KELVIN CHAU Canadian Food Inspection Agency, 1124 Finch Ave. W, Unit 2, Toronto, ON, Canada M3J 2E2 TIMOTHY A. DICKINSON Green Plant Herbarium (TRT), Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, ON, Canada M5S 2C6, and Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, ON, Canada M5S 3B2 ABSTRACT The impacts of ploidy level changes on plant physiology and ecology present interesting avenues of research, and many questions remain unanswered. -

Draft Final Report
Draft Saddle Mountain Open Space Preserve Management Plan Initial Study and Proposed Mitigated Negative Declaration Prepared for: Sonoma County Agricultural Preservation and Open Space District 747 Mendocino Avenue Santa Rosa, CA 95401 Prepared by: Prunuske Chatham, Inc. 400 Morris St., Suite G Sebastopol, CA 95472 March 2019 This page is intentionally blank. Sonoma County Agricultural Preservation and Open Space District March 2019 Saddle Mountain Open Space Preserve Management Plan Initial Study/Proposed Mitigated Negative Declaration Table of Contents Page 1 Project Information ................................................................................................................................ 1 1.1 Introduction................................................................................................................................... 2 1.2 California Environmental Quality Act Requirements .................................................................... 3 1.2.1 Public and Agency Review ................................................................................................. 3 2 Project Description ................................................................................................................................. 4 2.1 Project Location and Setting ......................................................................................................... 4 2.2 Project Goals and Objectives ......................................................................................................... 4 -

Amelanchierspp. Family: Rosaceae Serviceberry
Amelanchier spp. Family: Rosaceae Serviceberry The genus Amelanchier contains about 16 species native to North America [5], Mexico [2], and Eurasia to northern Africa [4]. The word amelanchier is derived from the French common name amelanche of the European serviceberry, Amelanchier ovalis. Amelanchier alnifolia-juneberry, Pacific serviceberry, pigeonberry, rocky mountain servicetree, sarvice, sarviceberry, saskatoon, saskatoon serviceberry, western service, western serviceberry , western shadbush Amelanchier arborea-Allegheny serviceberry, apple shadbush, downy serviceberry , northern smooth shadbush, shadblow, shadblown serviceberry, shadbush, shadbush serviceberry Amelanchier bartramiana-Bartram serviceberry Amelanchier canadensis-American lancewood, currant-tree, downy serviceberry, Indian cherry, Indian pear, Indian wild pear, juice plum, juneberry, may cherry, sugar plum, sarvice, servicetree, shadberry, shadblow, shadbush, shadbush serviceberry, shadflower, thicket serviceberry Amelanchier florida-Pacific serviceberry Amelanchier interior-inland serviceberry Amelanchier sanguinea-Huron serviceberry, roundleaf juneberry, roundleaf serviceberry , shore shadbush Amelanchier utahensis-Utah serviceberry Distribution In North America throughout upper elevations and temperate forests. The Tree Serviceberry is a shrub or tree that reaches a height of 40 ft (12 m) and a diameter of 2 ft (0.6 m). It grows in many soil types and occurs from swamps to mountainous hillsides. It flowers in early spring, producing delicate white flowers, making -

Artemisia Californica Less
I. SPECIES Artemisia californica Less. [Updated 2017] NRCS CODE: Subtribe: Artemisiinae ARCA11 Tribe: Anthemideae (FEIS CODE: Family: Asteraceae ARCAL) Order: Asterales Subclass: Asteridae Class: Magnoliopsida flowering heads spring growth seedling, March 2009 juvenile plant photos A. Montalvo flowering plant, November 2005 mature plant with flower buds August 2010 A. Subspecific taxa None. Artemisia californica Less. var. insularis (Rydb.) Munz is now recognized as Artemisia nesiotica P.H. Raven (Jepson eFlora 2017). B. Synonyms Artemisia abrotanoides Nuttall; A. fischeriana Besser; A. foliosa Nuttall; Crossostephium californicum (Lessing) Rydberg (FNA 2017). C. Common name California sagebrush. The common name refers to its strong, sage-like aroma and endemism to California and Baja California. Other names include: coastal sage, coast sage, coast sagebrush (Painter 2016). D. Taxonomic relationships The FNA (2017) places this species in subgenus Artemisia . The molecular phylogeny of the genus has improved the understanding of relationships among the many species of Artemisia and has, at times, placed the species in subgenus Tridentadae; morphology of the inflorescences and flowers alone does not place this species with its closest relatives (Watson et al. 2002). The detailed phylogeny is not completely resolved (Hayat et al. 2009). E. Related taxa in region There are 18 species and a total of 31 taxa (including infrataxa) of Artemisia in southern California, all of which differ clearly from A. californica in habitat affinity, structure, or both (Munz 1974, Jepson eFlora 2017). Within subgenus Artemisia (as per FNA 2017), A. nesiotica from the Channel Islands is the most similar and was once considered part of A. californica ; it can be distinguished by its wider leaves with flat leaf margins (not rolled under). -

ECOLOGICAL and ECONOMIC IMPORTANCE of STUDYING PROPAGATION TECHNIQUES of COMMON HAWTHORN Crataegus Monogyna Jacq. G
СИБИРСКИЙ ЛЕСНОЙ ЖУРНАЛ. 2019. № 4. С. 63–67 UDC 581.16/581.6/581.9:634.17 ECOLOGICAL AND ECONOMIC IMPORTANCE OF STUDYING PROPAGATION TECHNIQUES OF COMMON HAWTHORN Crataegus monogyna Jacq. G. Özyurt, Z. Yücesan, N. Ak, E. Oktan, A. Ö. Üçler Karadeniz Technical University Trabzon, 61080 Turkey E-mail: [email protected], [email protected], [email protected], [email protected], [email protected] Received 11.04.2019 Climate change as a fact of global warming requires the development of different perspectives on the planning and implementation of sustainable forestry techniques. Increasing temperatures cause drought on a global basis. In connection with, this using drought tolerant species in afforestation work is of great importance. In recent years Crataegus L. species (hawthorn) are also involved in afforestation. One of these species, C. monogyna, is characterized by drought tolerance. Furthermore, C. monogyna is the most important nonwood forest product species of Turkey. Hawthorn is widely used in medicine (treatment of coronary heart diseases), and cosmetics industry, agriculture and animal husbandry and human nutrition. On the other hand, it is used in erosion control, afforestation, industrial energy resources and for landscaping. Economic and ecological contribution of hawthorn to the national economy is quite high. Therefore, determination of suitable generative and vegetative reproduction techniques and vast production of seedlings of hawthorn species are extremely important. The characteristics of generative and vegetative propagation of Crataegus are discussed. For generative propagation of hawthorn species, the most effective and suitable procedure is treatment of seeds in ash solution. For vegetative propagation in culture in vitro the growth induced by BA (benzyladenine) and IBA (indole butyric acid) hormones increases the rate of callus formation and rooting. -

Original Research Article
1 Original Research Article 2 3 THE MALOIDEAE (ROSACEAE) STRUCTURAL AND FUNCTIONAL FEATURES 4 DETERMINING PASSIVE IMMUNITY TO MYCOSIS 5 6 7 With the help of microscopic methods the leaves and fruits surface tissues of plants of four 8 genera of the Maloideae subfamily were screened: Malus Mill., Pyrus L., Cydonia Mill., 9 Mespilus L., as model objects, and attempts were made to explain the dependence of mycosis 10 damage on microstructural features. The species composition of fungi that cause damage to the 11 Maloideae leaves and fruits in the Russia southern regions is analyzed. It is established that 12 among pathogens with different types of parasitism there are common excitants, as well as 13 highly specialized, more represented on Mespilus germanica. Higher resistance to the complex 14 of fungal diseases, in comparison with apple and pear, was found in quince and medlar. This 15 stability at the initial stage of the pathological process is associated with structural features such 16 as micromorphology of the fruits and stomata cuticle in the abaxial leaves epidermis. The leaves 17 stomatal cracks of the medlar are narrow with raised outgrowths, on the surface of the fruit – the 18 layered structure of the cuticular layer. Quince has a powerful continuous cuticular cover. 19 Compared with Malus and Pyrus, Cydonia and Mespilus also have a large (30 % or more) 20 polyphenol content in the pericarp outer layer cells. In addition to the gender-specific differences 21 in the microstructure of the integumentary tissues and the content of polyphenols affecting the 22 resistance to pathogens at the stage of their penetration, general patterns of leaf surface 23 formation, such as hypostomacy, anomocytic stomata, folded microrelief of the cuticular surface, 24 and the presence of single and multicellular trichomes are noted. -
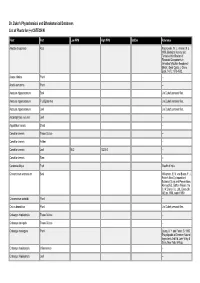
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN Plant Part Low PPM High PPM StdDev Reference Abutilon theophrasti Root Paszkowski, W. L., Kremer, R. J. 1988. Biological Activity and Tentative Identification of Flavonoid Components in Velvetleaf (Abutilon theophrasti Medik.) Seed Coats. J. Chem. Ecol., 14(7): 1573-1582. Acacia nilotica Plant -- Acacia decurrens Plant -- Aesculus hippocastanum Bark Jim Duke's personal files. Aesculus hippocastanum Fruit Epidermis Jim Duke's personal files. Aesculus hippocastanum Leaf Jim Duke's personal files. Arctostaphylos uva-ursi Leaf -- Aspalathus linearis Shoot -- Camellia sinensis Tissue Culture -- Camellia sinensis Anther -- Camellia sinensis Leaf 85.2 13200.0 -- Camellia sinensis Stem -- Ceratonia siliqua Fruit Wealth of India. Cinnamomum aromaticum Bark Williamson, E. M. and Evans, F. J., Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed., Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 362 pp, 1988, reprint 1989. Cinnamomum sieboldii Plant -- Cnicus benedictus Plant Jim Duke's personal files. Crataegus rhipidophylla Tissue Culture -- Crataegus laevigata Tissue Culture -- Crataegus monogyna Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Inflorescence -- Crataegus rhipidophylla Leaf -- Plant Part Low PPM High PPM StdDev Reference Crataegus laevigata Leaf -- Crataegus laevigata Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Bud -- Crataegus rhipidophylla Flower -- Crataegus laevigata Bud -- Crataegus laevigata Flower -- Croton lechleri Plant -- Eucalyptus globulus Leaf -- Fagopyrum esculentum Root -- Fagopyrum esculentum Tissue Culture -- Fallopia japonica Root 2.0 -- Geranium thunbergii Tissue Culture -- Ginkgo biloba Tissue Culture Jim Duke's personal files. -

Amelanchier Canadensis
AmelanchierAmelanchier canadensiscanadensis ShadblowShadblow serviceberry,serviceberry, CanadianCanadian serviceberry,serviceberry, DownyDowny serviceberry,serviceberry, Shadbush,Shadbush, Juneberry,Juneberry, ChuckleberryChuckleberry Amelanchier canadensis(Shadblow serviceberry) is native to South Canada and the eastern United States. The shrub was introduced in Europe in 1746. The Shadblow serviceberry grows up to 5 to 6 metres tall and wide with a finely branched, wide vase-shaped crown. Before the leaves start to sprout, the Amelanchier canadensisblooms bountifully racemes of white flowers that hang over slightly. In late July, it bears red-violet to blue-black fruits that are edible. The leaves are relatively large and bud in a lovely bronze-red. In the summer, the Shadblow serviceberry has green leaves with a grey-green underside. The autumn colours are yellow-orange to brown and less striking than those of other serviceberry shrubs. The species has a preference for sunlight and moist, humous, slightly acidic soil. It will tolerate a temporarily dry environment, as well as strong wind. That makes Amelanchier canadensis very suitable for use in containers and roof gardens. SEASONAL COLOURS jan feb mar apr mei jun jul aug sep okt nov dec TYPES OF PLANTING Tree types: fruit trees, solitary shrubs | Topiary on stem: multi-stem umbrella USE Location: park, central reservation, in containers, roof garden, large garden, small garden, patio, cemetery, countryside, ecological zone | Pavement: none, open | Planting concepts: Eco planting, -

Plant Collecting Expedition for Berry Crop Species Through Southeastern
Plant Collecting Expedition for Berry Crop Species through Southeastern and Midwestern United States June and July 2007 Glassy Mountain, South Carolina Participants: Kim E. Hummer, Research Leader, Curator, USDA ARS NCGR 33447 Peoria Road, Corvallis, Oregon 97333-2521 phone 541.738.4201 [email protected] Chad E. Finn, Research Geneticist, USDA ARS HCRL, 3420 NW Orchard Ave., Corvallis, Oregon 97330 phone 541.738.4037 [email protected] Michael Dossett Graduate Student, Oregon State University, Department of Horticulture, Corvallis, OR 97330 phone 541.738.4038 [email protected] Plant Collecting Expedition for Berry Crops through the Southeastern and Midwestern United States, June and July 2007 Table of Contents Table of Contents.................................................................................................................... 2 Acknowledgements:................................................................................................................ 3 Executive Summary................................................................................................................ 4 Part I – Southeastern United States ...................................................................................... 5 Summary.............................................................................................................................. 5 Travelog May-June 2007.................................................................................................... 6 Conclusions for part 1 ..................................................................................................... -

Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription
Hindawi BioMed Research International Volume 2018, Article ID 7627191, 10 pages https://doi.org/10.1155/2018/7627191 Research Article Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription Jiahui Sun ,1,2 Shuo Shi ,1,2,3 Jinlu Li,1,4 Jing Yu,1 Ling Wang,4 Xueying Yang,5 Ling Guo ,6 and Shiliang Zhou 1,2 1 State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China 2University of the Chinese Academy of Sciences, Beijing 100043, China 3College of Life Science, Hebei Normal University, Shijiazhuang 050024, China 4Te Department of Landscape Architecture, Northeast Forestry University, Harbin 150040, China 5Key Laboratory of Forensic Genetics, Institute of Forensic Science, Ministry of Public Security, Beijing 100038, China 6Beijing Botanical Garden, Beijing 100093, China Correspondence should be addressed to Ling Guo; [email protected] and Shiliang Zhou; [email protected] Received 21 September 2017; Revised 11 December 2017; Accepted 2 January 2018; Published 19 March 2018 Academic Editor: Fengjie Sun Copyright © 2018 Jiahui Sun et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Maleae consists of economically and ecologically important plants. However, there are considerable disputes on generic circumscription due to the lack of a reliable phylogeny at generic level. In this study, molecular phylogeny of 35 generally accepted genera in Maleae is established using 15 chloroplast regions. Gillenia isthemostbasalcladeofMaleae,followedbyKageneckia + Lindleya, Vauquelinia, and a typical radiation clade, the core Maleae, suggesting that the proposal of four subtribes is reasonable. -

Physicochemical, Nutritional and Health-Related Component Characterization of the Underutilized Mexican Serviceberry Fruit [Malacomeles Denticulata (Kunth) G
Original article Physicochemical, nutritional and health-related component characterization of the underutilized Mexican serviceberry fruit [Malacomeles denticulata (Kunth) G. N. Jones] María C. CAZARES-FRANCO1, Carlos RAMÍREZ-CHIMAL2, María G. HERRERA-HERNÁNDEZ3, Carlos A. NÚÑEZ-COLÍN4, 5 3 Miguel A. HERNÁNDEZ-MARTÍNEZ , Salvador H. GUZMÁN-MALDONADO * 1 Div. Cienc. Salud Ing., Physicochemical, nutritional and health-related component characterization Campus Celaya-Salvatierra, of the underutilized Mexican serviceberry fruit [Malacomeles denticulata Univ. Guanajuato, (Kunth) G. N. Jones]. Av. Juan Paplo II s/n, Abstract – Introduction. The nutritional and functional qualities of wild and cultivated Celaya, Gto, México Mexican serviceberry have not yet been reported. This species could have similar potential for com- 2 Univ. Politéc. Guanajuato. mercialization to that of Saskatoon berry (Amelanchier alnifolia Nutt.). Materials and methods. Wild Ave. Univ. Norte s/n. and cultivated fruits at two maturity stages were assessed for CIE Lab color, fruit size, titratable acidity Juan Alonso, Cortazar, and total soluble solids. Also, chemical composition and mineral contents were determined. In addi- tion, vitamin C and simple phenols were assessed. Total soluble phenols, condensed tannins and Guanajuato, México, CP 38483 anthocyanins as well as Trolox antioxidant activity and oxygen radical antioxidant activity were deter- 3 Unidad Biotecnol., Campo mined. Results. Fruit size, titratable acidity, total soluble solids, iron and simple phenols were higher Exp. Bajío (INIFAP), km. 6.5 in fruits of cultivated plants than in those of wild plants. Total fiber, calcium, vitamin C, total soluble Carretera Celaya, San Miguel phenols and condensed tannins were higher in wild fruits. Wild and cultivated serviceberry showed higher Trolox antioxidant activity compared with oxygen radical antioxidant activity. -

Principles and Practice of Forest Landscape Restoration Case Studies from the Drylands of Latin America Edited by A.C
Principles and Practice of Forest Landscape Restoration Case studies from the drylands of Latin America Edited by A.C. Newton and N. Tejedor About IUCN IUCN, International Union for Conservation of Nature, helps the world find pragmatic solutions to our most pressing environment and development challenges. IUCN works on biodiversity, climate change, energy, human livelihoods and greening the world economy by supporting scientific research, managing field projects all over the world, and bringing governments, NGOs, the UN and companies together to develop policy, laws and best practice. IUCN is the world’s oldest and largest global environmental organization, with more than 1,000 government and NGO members and almost 11,000 volunteer experts in some 160 countries. IUCN’s work is supported by over 1,000 staff in 60 offices and hundreds of partners in public, NGO and private sectors around the world. www.iucn.org Principles and Practice of Forest Landscape Restoration Case studies from the drylands of Latin America Principles and Practice of Forest Landscape Restoration Case studies from the drylands of Latin America Edited by A.C. Newton and N. Tejedor This book is dedicated to the memory of Margarito Sánchez Carrada, a student who worked on the research project described in these pages. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN or the European Commission concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries.