Whole Plant Based Treatment of Hypercholesterolemia with Crataegus Laevigata in a Zebrafish Model Robert M Littleton1, Matthew Miller1,2 and Jay R Hove1,2*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ECOLOGICAL and ECONOMIC IMPORTANCE of STUDYING PROPAGATION TECHNIQUES of COMMON HAWTHORN Crataegus Monogyna Jacq. G
СИБИРСКИЙ ЛЕСНОЙ ЖУРНАЛ. 2019. № 4. С. 63–67 UDC 581.16/581.6/581.9:634.17 ECOLOGICAL AND ECONOMIC IMPORTANCE OF STUDYING PROPAGATION TECHNIQUES OF COMMON HAWTHORN Crataegus monogyna Jacq. G. Özyurt, Z. Yücesan, N. Ak, E. Oktan, A. Ö. Üçler Karadeniz Technical University Trabzon, 61080 Turkey E-mail: [email protected], [email protected], [email protected], [email protected], [email protected] Received 11.04.2019 Climate change as a fact of global warming requires the development of different perspectives on the planning and implementation of sustainable forestry techniques. Increasing temperatures cause drought on a global basis. In connection with, this using drought tolerant species in afforestation work is of great importance. In recent years Crataegus L. species (hawthorn) are also involved in afforestation. One of these species, C. monogyna, is characterized by drought tolerance. Furthermore, C. monogyna is the most important nonwood forest product species of Turkey. Hawthorn is widely used in medicine (treatment of coronary heart diseases), and cosmetics industry, agriculture and animal husbandry and human nutrition. On the other hand, it is used in erosion control, afforestation, industrial energy resources and for landscaping. Economic and ecological contribution of hawthorn to the national economy is quite high. Therefore, determination of suitable generative and vegetative reproduction techniques and vast production of seedlings of hawthorn species are extremely important. The characteristics of generative and vegetative propagation of Crataegus are discussed. For generative propagation of hawthorn species, the most effective and suitable procedure is treatment of seeds in ash solution. For vegetative propagation in culture in vitro the growth induced by BA (benzyladenine) and IBA (indole butyric acid) hormones increases the rate of callus formation and rooting. -
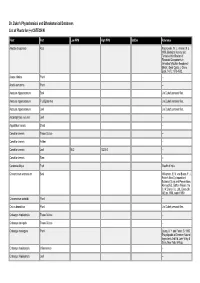
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN Plant Part Low PPM High PPM StdDev Reference Abutilon theophrasti Root Paszkowski, W. L., Kremer, R. J. 1988. Biological Activity and Tentative Identification of Flavonoid Components in Velvetleaf (Abutilon theophrasti Medik.) Seed Coats. J. Chem. Ecol., 14(7): 1573-1582. Acacia nilotica Plant -- Acacia decurrens Plant -- Aesculus hippocastanum Bark Jim Duke's personal files. Aesculus hippocastanum Fruit Epidermis Jim Duke's personal files. Aesculus hippocastanum Leaf Jim Duke's personal files. Arctostaphylos uva-ursi Leaf -- Aspalathus linearis Shoot -- Camellia sinensis Tissue Culture -- Camellia sinensis Anther -- Camellia sinensis Leaf 85.2 13200.0 -- Camellia sinensis Stem -- Ceratonia siliqua Fruit Wealth of India. Cinnamomum aromaticum Bark Williamson, E. M. and Evans, F. J., Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed., Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 362 pp, 1988, reprint 1989. Cinnamomum sieboldii Plant -- Cnicus benedictus Plant Jim Duke's personal files. Crataegus rhipidophylla Tissue Culture -- Crataegus laevigata Tissue Culture -- Crataegus monogyna Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Inflorescence -- Crataegus rhipidophylla Leaf -- Plant Part Low PPM High PPM StdDev Reference Crataegus laevigata Leaf -- Crataegus laevigata Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Bud -- Crataegus rhipidophylla Flower -- Crataegus laevigata Bud -- Crataegus laevigata Flower -- Croton lechleri Plant -- Eucalyptus globulus Leaf -- Fagopyrum esculentum Root -- Fagopyrum esculentum Tissue Culture -- Fallopia japonica Root 2.0 -- Geranium thunbergii Tissue Culture -- Ginkgo biloba Tissue Culture Jim Duke's personal files. -

Crataegus Laevigata 'Crimson Cloud' 'Crimson Cloud' English Hawthorn
Fact Sheet ST-211 November 1993 Crataegus laevigata ‘Crimson Cloud’ ‘Crimson Cloud’ English Hawthorn1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Crimson Cloud (also known as ‘Superba’) English Hawthorn grows rapidly in a pyramidal form to about 20 feet, then the crown expands to become oval or irregular (Fig. 1). The tree tolerates most soils, growing well in clay, but prefers heavy, dry loam. The main ornamental feature is white and red flowers borne in spring which together give the tree a deep pink color. Fruits are red and quite showy but do not cover the tree. Though quite ornamental, Hawthorns are susceptible to insect and disease problems. Branching habit is decidedly drooping and care should be given when locating this tree near pedestrian or vehicular traffic. GENERAL INFORMATION Figure 1. Middle-aged ‘Crimson Cloud’ English Hawthorn. Scientific name: Crataegus laevigata ‘Crimson Cloud’ Availability: grown in small quantities by a small Pronunciation: kruh-TEE-gus lee-vih-GAY-tuh number of nurseries Common name(s): ‘Crimson Cloud’ English Hawthorn DESCRIPTION Family: Rosaceae USDA hardiness zones: 4B through 8 (Fig. 2) Height: 20 to 25 feet Origin: not native to North America Spread: 15 to 25 feet Uses: Bonsai; espalier; wide tree lawns (>6 feet Crown uniformity: irregular outline or silhouette wide); medium-sized tree lawns (4-6 feet wide); Crown shape: oval; pyramidal recommended for buffer strips around parking lots or Crown density: moderate for median strip plantings in the highway; reclamation Growth rate: medium plant; screen; narrow tree lawns (3-4 feet wide); Texture: fine specimen; sidewalk cutout (tree pit); residential street tree; tree has been successfully grown in urban areas where air pollution, poor drainage, compacted soil, and/or drought are common 1. -

(Rosaceae), I. Differentiation of Mespilus and Crataegus
Phytotaxa 257 (3): 201–229 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2016 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.257.3.1 STUDIES IN MESPILUS, CRATAEGUS, AND ×CRATAEMESPILUS (ROSACEAE), I. DIFFERENTIATION OF MESPILUS AND CRATAEGUS, EXPANSION OF ×CRATAEMESPILUS, WITH SUPPLEMENTARY OBSERVATIONS ON DIFFERENCES BETWEEN THE CRATAEGUS AND AMELANCHIER CLADES JAMES B. PHIPPS Department of Biology, The University of Western Ontario, London, Ontario N6A 5B7, Canada; email: [email protected] Abstract The paper argues the position for retaining a monotypic Mespilus, i.e., in the sense of M. germanica, the medlar. Recent cladistic papers lend support for Mespilus being sister to Crataegus, and there is a clear morphological distinction from Cra- taegus, emphasized by adaptation to carnivore frugivory. Mespilus secured, the paper then treats each of the known hybrids between Mespilus and Crataegus, making the new combination Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps. Keywords: Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps comb. nov.; inflorescence position; medlar; Mespilus a folk-genus; Mespilus distinct from Crataegus; Rosaceae; taxonomic history of Mespilus Introduction The author has a long-standing interest in generic delimitation in the Maloid genera of the Rosaceae (Maleae Small, formerly Maloideae C. Weber, Pyrinae Dumort.), as shown particularly in a series of papers with K. Robertson, J. Rohrer, and P.G. Smith (Phipps et al. 1990, 1991; Robertson at al. 1991, 1992; Rohrer at al. 1991, 1994) which treated all 28 genera of Maleae as recognised by us. There is also a revisionary treatment of New World Heteromeles M.J. -

Crataegus ×Media 'Paul's Scarlet'
All the knowledge. Almost all of the trees. https://www.vdberk.co.uk/trees/crataegus-media-paul-s-scarlet/ Crataegus ×media 'Paul's Scarlet' Height 6 - 8 (10) m Crown broad spherical, later rounded, half-open crown, capricious growing Bark and branches bark brownish-grey, flaking off in platelets, twigs thorny Leaf ovoid, 3/5-lobed, dark green, 3 - 6 cm Flowers red double flowers in umbels, May-June Fruits none, fruitless cultivar Spines/thorns Yes Toxicity usually not toxic to people, (large) pets and livestock Soil type makes few demands, preferably not too dry Paving tolerates paving Winter hardiness zone 5a (-28,8 to -26,1 °C) Wind resistance moderate Other resistances resistant to frost (WH 1 - 6) Fauna tree resistant to frost (WH 1 - 6), valuable for bees (honey plant) Application avenues and broad streets, parks, squares, cemeteries, large gardens, small gardens Shape clearstem tree, multi-stem treem Origin Wm. Paul, England, 1860 Synonyms Crataegus laevigata 'Paul's Scarlet' Sometimes grown as a shrub but mostly sold as a tree. The sturdy lateral branches that grow outwards create a more or less rounded open type of crown. It can eventually attain a maximum width of 6 to 7 m. The trunk and branches are grey. The branches are less thorny than those of the species. The thorns are approx. 2.5 cm long and viciously sharp. The dark green leaves are lighter on the underside. The entire tree is covered with blossom in the spring. The flowers turn from even to pale red. The tough hard roots go deep and spread wide. -

Trees to Avoid Planting in the Midwest and Some Excellent Alternatives
Trees to Avoid Planting in the Midwest and Some Excellent Alternatives Dr. Laura G. Jull Dept. of Horticulture, UW-Madison Trees provide us with many environmental, aesthetic, functional, and economic benefits. Tree selection is one of the most important considerations when a homeowner, nurserymen, or landscaper is deciding what species to grow or plant. Many questions need to be answered including size, location, site characteristics, aesthetic features, pest susceptibility, hardiness, and maintenance considerations. Some trees can become a maintenance headache due to their inherent pest problems or lack of structural integrity. The trees represented in this story have not generally performed well in urban and suburban areas of the Midwest. Some are susceptible to insects and diseases, and some have severe structural problems such as being weak- wooded or prone to girdling roots or included bark formation. Others have cultural problems such as intolerance to high pH, road salt, drought, and poor drainage. A few tree species are invasive and should be avoided near sensitive areas or seed dispersal into woodlands could occur. Some of these trees may do quite well in other parts of the U.S., so my intention is not to apply a blanket statement for all these trees to all situations. Invasiveness and pest susceptibility can vary geographically. The article is based on more than 25 years of field experience and data collected from numerous states’ plant disease and insect diagnostic clinics, and conversations with arborists, nurseries, landscapers, and extension personnel. There are alternative species that can be used and are mentioned here. These alternative tree species have performed well in USDA Cold Hardiness Zone 4b. -

The Plant List
the list A Companion to the Choosing the Right Plants Natural Lawn & Garden Guide a better way to beautiful www.savingwater.org Waterwise garden by Stacie Crooks Discover a better way to beautiful! his plant list is a new companion to Choosing the The list on the following pages contains just some of the Right Plants, one of the Natural Lawn & Garden many plants that can be happy here in the temperate Pacific T Guides produced by the Saving Water Partnership Northwest, organized by several key themes. A number of (see the back panel to request your free copy). These guides these plants are Great Plant Picks ( ) selections, chosen will help you garden in balance with nature, so you can enjoy because they are vigorous and easy to grow in Northwest a beautiful yard that’s healthy, easy to maintain and good for gardens, while offering reasonable resistance to pests and the environment. diseases, as well as other attributes. (For details about the GPP program and to find additional reference materials, When choosing plants, we often think about factors refer to Resources & Credits on page 12.) like size, shape, foliage and flower color. But the most important consideration should be whether a site provides Remember, this plant list is just a starting point. The more the conditions a specific plant needs to thrive. Soil type, information you have about your garden’s conditions and drainage, sun and shade—all affect a plant’s health and, as a particular plant’s needs before you purchase a plant, the a result, its appearance and maintenance needs. -

Advantageous Extraction, Cleanup, and UHPLC-MS/MS Detection of Patulin Mycotoxin in Dietary Supplements and Herbal Blends Containing Hawberry from Crataegus Spp
Hindawi Journal of Analytical Methods in Chemistry Volume 2019, Article ID 2159097, 13 pages https://doi.org/10.1155/2019/2159097 Research Article Advantageous Extraction, Cleanup, and UHPLC-MS/MS Detection of Patulin Mycotoxin in Dietary Supplements and Herbal Blends Containing Hawberry from Crataegus spp. Anna Przybylska ,1 Grzegorz Bazylak ,1 Robert Kosicki,2 Iwona Altyn ,2 Magdalena Twaruzek ,2 Jan Grajewski ,2 and Anna Soltys-Lelek3 1Department of Pharmaco-Bromatology and Molecular Nutrition, Faculty of Pharmacy, Collegium Medicum, Nicolaus Copernicus University, Jagiellonska 13, PL-85067 Bydgoszcz, Poland 2Department of Physiology and Toxicology, Institute of Experimental Biology, Faculty of Natural Sciences, Kazimierz Wielki University, Chodkiewicza 30, PL-85064 Bydgoszcz, Poland 3Ojcow National Park, Ojcow 9, PL-32045 Suloszowa, Poland Correspondence should be addressed to Grzegorz Bazylak; [email protected] Received 14 October 2018; Revised 21 December 2018; Accepted 13 January 2019; Published 6 February 2019 Academic Editor: Nu´ria Fontanals Copyright © 2019 Anna Przybylska et al. /is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Patulin (PAT) is a highly genotoxic mycotoxin still found as the common contaminant of various kinds of spoiled fruits and related commodities which are often endorsed as the health-enhancing products. /us, a fast and convenient liquid-solid extraction followed by a solid-phase cleanup with the MycoSep 228 AflaPat multifunctional column was used for the highly efficient isolation of PAT with an average recovery of 112.7% from® commercial dietary supplements and herbal blends formulated with dried hawberry. -

2986-2991, 2012 Issn 1995-0756
2986 Advances in Environmental Biology, 6(11): 2986-2991, 2012 ISSN 1995-0756 This is a refereed journal and all articles are professionally screened and reviewed ORIGINAL ARTICLE A study of microsporogenesis and pollen morphology in Crataegus babakhanloui (Rosaceae) Rahmani Hamideh, Majd Ahmad, Arbabian Sedigheh, Sharfnia Fariba, Mehrabian Sedigheh Department of Biology, North Tehran Branch, Islamic Azad University, Tehran, Iran Rahmani Hamideh, Majd Ahmad, Arbabian Sedigheh, Sharfnia Fariba, Mehrabian Sedigheh; A study of microsporogenesis and pollen morphology in Crataegus babakhanloui (Rosaceae) ABSTRACT In this study, microsporogenesis and pollen morphology of Crataegus babakhanloui were studied. The flowers, in different developmental stages, were removed, fixed in Formalin -glacial acetic acid- alcohol (FAA), stored in 70% ethanol, embedded in paraffin and then sliced at 8-10 μm by rotary microtome. Staining was carried out by periodic Acid Schiff (PAS) and contrasted with hematoxylin. Scanning electron microscope (SEM) was used to analyze the mature pollen grains. The results indicated that anthers wall development followed the dicotyledonous type and were tetrasporangiate witch composed of epidermal layer, endothecium layer, two rows of middle layers and then tapetum layer. Tapetum was dimorphic in early and late process. Microspore tetrads are tetrahedral and Pollen grains are shed at bicellular stage. Pollen grains are tricolporate, medium size and prolate. Exine sculpturing is striate with perforations on grain surface. Key words: Crataegus babakhanloui, Microsporogenesis, Pollen grain. Introduction in diameter, almost spherical, purplish-black and dusty with 3-4 stones. Hawthorn (Crataegus spp.) ornamentally and Hawthorns provide food and shelter for many medically has a big name in science history. The species of birds and mammals, and the flowers are genus Crataegus belongs to the subfamily Maloideae important for many nectar-feeding insects [3,17]. -

Crataegus (Hawthorn)
nysipm.cornell.edu 2019 Search for this title at the NYSIPM Publications collection: ecommons.cornell.edu/handle/1813/41246 Disease and Insect Resistant Ornamental Plants Mary Thurn, Elizabeth Lamb, and Brian Eshenaur New York State Integrated Pest Management Program, Cornell University CRATAEGUS Hawthorn pixabay.com Crataegus is a large genus of shrubs and small trees in the rose family commonly known as hawthorn. This popular ornamental has showy pink or white flowers in spring and colorful berry-like fruit. Some species also have long thorns that provide protection for wildlife but may be a hazard in the landscape–thornless cultivars are available. Like other rosaceous plants, hawthorns are sus- ceptible to a number of diseases including fire blight, scab, leaf spot and several types of rust. Insect pests include lace bugs and leaf miners. DISEASES Cedar Rust diseases on hawthorn, which include hawthorn rust and quince rust, are caused by sev- eral fungi in the genus Gymnosporangium that spend part of their life cycle on Eastern red cedar (Juni- perus virginiana) and other susceptible junipers, and another part of their life cycle on plants in the rose family, especially Malus and Crataegus. Since two hosts are required for these fungi to complete their life cycle, one way to reduce disease problems is to avoid planting alternate hosts near each other. Hawthorn Rust, caused by Gymnosporangium globosum, is a significant concern for Crataegus spp. in the Northeast (7). Hawthorns are the main broadleaved host for this rust, and yellow-orange leaf spots are the most common symptom. (8). With severe infections, foliage may turn bright yellow and drop prematurely (15). -

JBG Plant List
Jensen Botanical Garden Plant List When you are at the garden, you will see metal signs with labels. each will have a number and the common name of the plant. This list will give you more information. 1.Valley Oak; Quercus lobata. Native to the interior valleys and sierra foothills. Deciduous. California's mightiest oak, often reaching 70 feet or more, with equal or greater spread. This was on the property when Mr. Jensen bought it and has been estimated to be over 400 years old. 2. Rose; “Day Dream” Shrub. 18-24 inches high and 24-36 inches wide is normal. JBG ones are well established and very happy, so they exceed the normal size. Medium pink flower with a slight fragrance, multiple flowers on each stem. Blooms repeatedly - prune frequently to encourage new blooms. 3. Japanese Maple, Acer palatum “Sangu Kaku” or Senkaki. Vigorous, upright treelike growth. Fall foliage yellow, tinted rose. Twigs, branches striking coral red. 4. Flowering Maple, Abutilon. Evergreen vine shrubs. Part shade. Grown daily for bell shaped flowers. Bloom April-June, but some bloom year round. Available in white, yellow, pink, and red. Attracts bees and hummingbirds. 5. Sage, Salvia microphylla. Blooms all through the hottest summers, attracting hummingbirds and butterflies. Drought tolerant once established. Plant in full sun, grows quickly to 3 foot by 3 foot. 6. Interior live oak; Quercus wislizenii. Native to the Sierra foothills and east side of California’s central valley. Evergreen. Grows 30 - 75 feet high; often broader than high. Oblong, glossy green leaves to 4 inches long, smooth or spiny edged. -

Crataegus Spp.) Fruits Species for Potential Use in Food Applications
foods Article Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications Abolfazl Alirezalu 1 , Nima Ahmadi 2,*, Peyman Salehi 3, Ali Sonboli 3, Kazem Alirezalu 4, Amin Mousavi Khaneghah 5, Francisco J. Barba 6 , Paulo E.S. Munekata 7 and Jose M. Lorenzo 7,* 1 Department of Horticultural Sciences, Faculty of Agriculture, Urmia University, Urmia 5756151818, Iran; [email protected] 2 Department of Horticultural Sciences, Faculty of Agriculture, Tarbiat Modares University, Tehran 1411713116, Iran 3 Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran 1983969411, Iran; [email protected] (P.S.); [email protected] (A.S.) 4 Department of Food Science and Technology, Ahar Faculty of Agriculture and Natural Resources, University of Tabriz, Tabriz, Iran; [email protected] 5 Department of Food Science, Faculty of Food Engineering, University of Campinas (UNICAMP), Campinas, 13083-862 São Paulo, Brazil; [email protected] 6 Nutrition and Food Science Area, Preventive Medicine and Public Health, Food Science, Toxicology and Forensic Medicine Department, Faculty of Pharmacy, Universitat de València, Avda.Vicent Andrés Estellés, s/n, 46100 Burjassot, València, Spain; [email protected] 7 Centro Tecnológico de la Carne de Galicia, rúa Galicia n◦ 4, Parque Tecnológico de Galicia, San Cibrao das Viñas, 32900 Ourense, Spain; [email protected] * Correspondence: [email protected] (N.A.); [email protected] (J.M.L.); Tel.: +98 21 48292088 (N.A.); +34-988548277 (J.M.L.) Received: 11 March 2020; Accepted: 1 April 2020; Published: 4 April 2020 Abstract: Hawthorn belongs to the Crataegus genus of the Rosaceae family and is an important medicinal plant.