Crataegus Spp.) Fruits Species for Potential Use in Food Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Seeds and Plants Imported
Issued November 9,1915. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY SEEDS AND PLANTS IMPORTED OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM APRIL 1 " " TO JUNE 30,1913. " , r (No. 35; Nos. 35136 TO 3566^..,--"•-****"*"' WASHINGTON: GOVERNMENT PRINTING OFFICE. 1915. Issued November 9,1915. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM APRIL 1 TO JUNE 30,1913. (No. 35; Nos. 35136 TO 35666.) WASHINGTON: GOVERNMENT PRINTING OFFICE. 1915. BUREAU OF PLANT INDUSTRY. Chief of Bureau, WILLIAM A. TAYLOR. Assistant Chief of Bureau, KARL F. KELLERMAN. Officer in Charge of Publications, J. E. ROCKWELL. Chief Clerk, JAMES E. JONES. FOREIGN SEED AND PLANT INTRODUCTION. SCIENTIFIC STAFF. David Fairchild, Agricultural Explorer in Charge. P. H. Dorsett, Plant Introducer, in Charge of Plant Introduction Field Stations. Peter Bisset, Plant Introducer, in Charge of Foreign Plant Distribution. Frank N. Meyer and Wilson Popenoe, Agricultural Explorers. H. C. Skeels, S. C. Stuntz, and R. A. Young, Botanical Assistants. Allen M. Groves, Nathan Menderson, and Glen P. Van Eseltine, Assistants. Robert L. Beagles, Superintendent, Plant Introduction Field Station, Chico, Cal. Edward Simmonds, Superintendent, Subtropical Plant Introduction Field Station, Miami, Fla. John M. Rankin, Superintendent, Yarrow Plant Introduction Field Station, Rockville, Md. E. R. Johnston, Assistant in Charge, Plant Introduction Field Station, Brooksville, Fla. Edward Goucher and H. Klopfer, Plant Propagators. Collaborators: Aaron Aaronsohn, Director, Jewish Agricultural Experimental Station, Haifa, Palestine; Thomas W. -

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

1 Acanthus Dioscoridis Acanthaceae 2 Blepharis Persica Acanthaceae 3
Row Species Name Family 1 Acanthus dioscoridis Acanthaceae 2 Blepharis persica Acanthaceae 3 Acer mazandaranicum Aceraceae 4 Acer monspessulanum subsp. persicum Aceraceae 5 Acer monspessulanum subsp. assyriacum Aceraceae 6 Acer monspessulanum subsp. cinerascens Aceraceae 7 Acer monspessulanum subsp. turcomanicum Aceraceae 8 Acer tataricum Aceraceae 9 Acer campestre Aceraceae 10 Acer cappadocicum Aceraceae 11 Acer monspessulanum subsp. ibericum Aceraceae 12 Acer hyrcanum Aceraceae 13 Acer platanoides Aceraceae 14 Acer velutinum Aceraceae 15 Aizoon hispanicum Aizoaceae 16 Mesembryanthemum nodiflorum Aizoaceae 17 Sesuvium verrucosum Aizoaceae 18 Zaleya govindia Aizoaceae 19 Aizoon canariense Aizoaceae 20 Alisma gramineum Alismataceae 21 Damasonium alisma Alismataceae 22 Alisma lanceolatum Alismataceae 23 Alisma plantago-aquatica Alismataceae 24 Sagittaria trifolia Alismataceae 25 Allium assadii Alliaceae Row Species Name Family 26 Allium breviscapum Alliaceae 27 Allium bungei Alliaceae 28 Allium chloroneurum Alliaceae 29 Allium ellisii Alliaceae 30 Allium esfandiarii Alliaceae 31 Allium fedtschenkoi Alliaceae 32 Allium hirtifolium Alliaceae 33 Allium kirindicum Alliaceae 34 Allium kotschyi Alliaceae 35 Allium lalesaricum Alliaceae 36 Allium longivaginatum Alliaceae 37 Allium minutiflorum Alliaceae 38 Allium shelkovnikovii Alliaceae 39 Allium subnotabile Alliaceae 40 Allium subvineale Alliaceae 41 Allium wendelboi Alliaceae 42 Nectaroscordum koelzii Alliaceae 43 Allium akaka Alliaceae 44 Allium altissimum Alliaceae 45 Allium ampeloprasum subsp. -

The Metabolites of the Dietary Flavonoid Quercetin Possess Potent Antithrombotic Activity, and Interact with Aspirin to Enhance Antiplatelet Effects
The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity, and interact with aspirin to enhance antiplatelet effects Article Published Version Creative Commons: Attribution 4.0 (CC-BY) Open Access Stainer, A. R., Sasikumar, P., Bye, A. P., Unsworth, A. P., Holbrook, L. M., Tindall, M., Lovegrove, J. A. and Gibbins, J. M. (2019) The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity, and interact with aspirin to enhance antiplatelet effects. TH Open, 3 (3). e244- e258. ISSN 2512-9465 doi: https://doi.org/10.1055/s-0039- 1694028 Available at http://centaur.reading.ac.uk/85495/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.1055/s-0039-1694028 Publisher: Thieme All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Published online: 30.07.2019 THIEME e244 Original Article The Metabolites of the Dietary Flavonoid Quercetin Possess Potent Antithrombotic Activity, and Interact with Aspirin to Enhance Antiplatelet Effects Alexander R. Stainer1 Parvathy Sasikumar1,2 Alexander P. Bye1 Amanda J. Unsworth1,3 Lisa M. Holbrook1,4 Marcus Tindall5 Julie A. Lovegrove6 Jonathan M. Gibbins1 1 Institute for Cardiovascular and Metabolic Research, School of Address for correspondence Jonathan M. -

ECOLOGICAL and ECONOMIC IMPORTANCE of STUDYING PROPAGATION TECHNIQUES of COMMON HAWTHORN Crataegus Monogyna Jacq. G
СИБИРСКИЙ ЛЕСНОЙ ЖУРНАЛ. 2019. № 4. С. 63–67 UDC 581.16/581.6/581.9:634.17 ECOLOGICAL AND ECONOMIC IMPORTANCE OF STUDYING PROPAGATION TECHNIQUES OF COMMON HAWTHORN Crataegus monogyna Jacq. G. Özyurt, Z. Yücesan, N. Ak, E. Oktan, A. Ö. Üçler Karadeniz Technical University Trabzon, 61080 Turkey E-mail: [email protected], [email protected], [email protected], [email protected], [email protected] Received 11.04.2019 Climate change as a fact of global warming requires the development of different perspectives on the planning and implementation of sustainable forestry techniques. Increasing temperatures cause drought on a global basis. In connection with, this using drought tolerant species in afforestation work is of great importance. In recent years Crataegus L. species (hawthorn) are also involved in afforestation. One of these species, C. monogyna, is characterized by drought tolerance. Furthermore, C. monogyna is the most important nonwood forest product species of Turkey. Hawthorn is widely used in medicine (treatment of coronary heart diseases), and cosmetics industry, agriculture and animal husbandry and human nutrition. On the other hand, it is used in erosion control, afforestation, industrial energy resources and for landscaping. Economic and ecological contribution of hawthorn to the national economy is quite high. Therefore, determination of suitable generative and vegetative reproduction techniques and vast production of seedlings of hawthorn species are extremely important. The characteristics of generative and vegetative propagation of Crataegus are discussed. For generative propagation of hawthorn species, the most effective and suitable procedure is treatment of seeds in ash solution. For vegetative propagation in culture in vitro the growth induced by BA (benzyladenine) and IBA (indole butyric acid) hormones increases the rate of callus formation and rooting. -
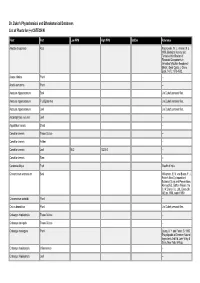
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for (+)-CATECHIN Plant Part Low PPM High PPM StdDev Reference Abutilon theophrasti Root Paszkowski, W. L., Kremer, R. J. 1988. Biological Activity and Tentative Identification of Flavonoid Components in Velvetleaf (Abutilon theophrasti Medik.) Seed Coats. J. Chem. Ecol., 14(7): 1573-1582. Acacia nilotica Plant -- Acacia decurrens Plant -- Aesculus hippocastanum Bark Jim Duke's personal files. Aesculus hippocastanum Fruit Epidermis Jim Duke's personal files. Aesculus hippocastanum Leaf Jim Duke's personal files. Arctostaphylos uva-ursi Leaf -- Aspalathus linearis Shoot -- Camellia sinensis Tissue Culture -- Camellia sinensis Anther -- Camellia sinensis Leaf 85.2 13200.0 -- Camellia sinensis Stem -- Ceratonia siliqua Fruit Wealth of India. Cinnamomum aromaticum Bark Williamson, E. M. and Evans, F. J., Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed., Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 362 pp, 1988, reprint 1989. Cinnamomum sieboldii Plant -- Cnicus benedictus Plant Jim Duke's personal files. Crataegus rhipidophylla Tissue Culture -- Crataegus laevigata Tissue Culture -- Crataegus monogyna Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Inflorescence -- Crataegus rhipidophylla Leaf -- Plant Part Low PPM High PPM StdDev Reference Crataegus laevigata Leaf -- Crataegus laevigata Plant Leung, A. Y. and Foster, S. 1995. Encyclopedia of Common Natural Ingredients 2nd Ed. John Wiley & Sons, New York. 649 pp. Crataegus rhipidophylla Bud -- Crataegus rhipidophylla Flower -- Crataegus laevigata Bud -- Crataegus laevigata Flower -- Croton lechleri Plant -- Eucalyptus globulus Leaf -- Fagopyrum esculentum Root -- Fagopyrum esculentum Tissue Culture -- Fallopia japonica Root 2.0 -- Geranium thunbergii Tissue Culture -- Ginkgo biloba Tissue Culture Jim Duke's personal files. -

Flavonoid Glucodiversification with Engineered Sucrose-Active Enzymes Yannick Malbert
Flavonoid glucodiversification with engineered sucrose-active enzymes Yannick Malbert To cite this version: Yannick Malbert. Flavonoid glucodiversification with engineered sucrose-active enzymes. Biotechnol- ogy. INSA de Toulouse, 2014. English. NNT : 2014ISAT0038. tel-01219406 HAL Id: tel-01219406 https://tel.archives-ouvertes.fr/tel-01219406 Submitted on 22 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Last name: MALBERT First name: Yannick Title: Flavonoid glucodiversification with engineered sucrose-active enzymes Speciality: Ecological, Veterinary, Agronomic Sciences and Bioengineering, Field: Enzymatic and microbial engineering. Year: 2014 Number of pages: 257 Flavonoid glycosides are natural plant secondary metabolites exhibiting many physicochemical and biological properties. Glycosylation usually improves flavonoid solubility but access to flavonoid glycosides is limited by their low production levels in plants. In this thesis work, the focus was placed on the development of new glucodiversification routes of natural flavonoids by taking advantage of protein engineering. Two biochemically and structurally characterized recombinant transglucosylases, the amylosucrase from Neisseria polysaccharea and the α-(1→2) branching sucrase, a truncated form of the dextransucrase from L. Mesenteroides NRRL B-1299, were selected to attempt glucosylation of different flavonoids, synthesize new α-glucoside derivatives with original patterns of glucosylation and hopefully improved their water-solubility. -

Towards an Updated Checklist of the Libyan Flora
Towards an updated checklist of the Libyan flora Article Published Version Creative Commons: Attribution 3.0 (CC-BY) Open access Gawhari, A. M. H., Jury, S. L. and Culham, A. (2018) Towards an updated checklist of the Libyan flora. Phytotaxa, 338 (1). pp. 1-16. ISSN 1179-3155 doi: https://doi.org/10.11646/phytotaxa.338.1.1 Available at http://centaur.reading.ac.uk/76559/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.11646/phytotaxa.338.1.1 Identification Number/DOI: https://doi.org/10.11646/phytotaxa.338.1.1 <https://doi.org/10.11646/phytotaxa.338.1.1> Publisher: Magnolia Press All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Phytotaxa 338 (1): 001–016 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.338.1.1 Towards an updated checklist of the Libyan flora AHMED M. H. GAWHARI1, 2, STEPHEN L. JURY 2 & ALASTAIR CULHAM 2 1 Botany Department, Cyrenaica Herbarium, Faculty of Sciences, University of Benghazi, Benghazi, Libya E-mail: [email protected] 2 University of Reading Herbarium, The Harborne Building, School of Biological Sciences, University of Reading, Whiteknights, Read- ing, RG6 6AS, U.K. -

Function-Structural Study of Anti-LDL-Oxidation Effects of Flavonoid Phytochemicals
Journal of Medicinal Plants Research Vol. 6(49), pp. 5895-5904, 25 December, 2012 Available online at http://www.academicjournals.org/JMPR DOI: 10.5897/JMPR12.313 ISSN 1996-0875 ©2012 Academic Journals Full Length Research Paper Function-structural study of anti-LDL-oxidation effects of flavonoid phytochemicals Yiwen Yao 1#, Shuqing Liu 2#, Chunmei Guo 3, Chunyan Zhang 3, Dachang Wu 3, Yixin Ren 2, Hong-Shan Yu 4, Ming-Zhong Sun 3 and Hailong Chen 5* 1Department of Otolaryngology, the First affiliated Hospital of Dalian Medical University, Dalian 116011, China. 2Department of Biochemistry, Dalian Medical University, Dalian 116044, China. 3Department of Biotechnology, Dalian Medical University, Dalian 116044, China. 4School of Bioengineering, Dalian Polytechnic University, Dalian 116034, China. 5Department of Surgery, the First Affiliated Hospital of Dalian Medical University, Dalian 116011, China Accepted 9 March, 2012 As a large group of polyphenolic phytochemicals with excellent anti-oxidation properties, the dietary flavonoid intakes have been shown to be negatively correlated with the incidence of coronary artery disease. Flavonoid compounds potentially inhibit the oxidation of low-density lipoprotein (Ox-LDL) that triggers the generation of a series of oxidation byproducts playing important roles in atherosclerosis development. The diverse inhibitory effects of different flavonoid phytochemicals on Ox-LDL might be closely associated with their intrinsic structures. In current work, we investigated the effects of eight flavonoid phytochemicals in high purity (>95%) with similar core structure on the susceptibility of LDL to Cu 2+ -induced oxidative modification. The results indicated that quercetin, rutin, isoquercitrin, hesperetin, naringenin, hesperidin, naringin and icariin could reduce the Cu 2+ -induced-LDL oxidation by 59.56 ±±±7.03, 46.53 ±±±2.09, 40.52 ±±±4.65, 22.67 ±±±1.68, 20.87 ±±±2.43, 12.34 ±±±2.09, 10.87 ±±±1.68 and 3.53 ±±±3.20%, respectively. -

Crataegus Laevigata 'Crimson Cloud' 'Crimson Cloud' English Hawthorn
Fact Sheet ST-211 November 1993 Crataegus laevigata ‘Crimson Cloud’ ‘Crimson Cloud’ English Hawthorn1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Crimson Cloud (also known as ‘Superba’) English Hawthorn grows rapidly in a pyramidal form to about 20 feet, then the crown expands to become oval or irregular (Fig. 1). The tree tolerates most soils, growing well in clay, but prefers heavy, dry loam. The main ornamental feature is white and red flowers borne in spring which together give the tree a deep pink color. Fruits are red and quite showy but do not cover the tree. Though quite ornamental, Hawthorns are susceptible to insect and disease problems. Branching habit is decidedly drooping and care should be given when locating this tree near pedestrian or vehicular traffic. GENERAL INFORMATION Figure 1. Middle-aged ‘Crimson Cloud’ English Hawthorn. Scientific name: Crataegus laevigata ‘Crimson Cloud’ Availability: grown in small quantities by a small Pronunciation: kruh-TEE-gus lee-vih-GAY-tuh number of nurseries Common name(s): ‘Crimson Cloud’ English Hawthorn DESCRIPTION Family: Rosaceae USDA hardiness zones: 4B through 8 (Fig. 2) Height: 20 to 25 feet Origin: not native to North America Spread: 15 to 25 feet Uses: Bonsai; espalier; wide tree lawns (>6 feet Crown uniformity: irregular outline or silhouette wide); medium-sized tree lawns (4-6 feet wide); Crown shape: oval; pyramidal recommended for buffer strips around parking lots or Crown density: moderate for median strip plantings in the highway; reclamation Growth rate: medium plant; screen; narrow tree lawns (3-4 feet wide); Texture: fine specimen; sidewalk cutout (tree pit); residential street tree; tree has been successfully grown in urban areas where air pollution, poor drainage, compacted soil, and/or drought are common 1. -

(12) Patent Application Publication (10) Pub. No.: US 2013/0243709 A1 Hanson Et Al
US 201302437.09A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0243709 A1 Hanson et al. (43) Pub. Date: Sep. 19, 2013 (54) NATURAL SUNSCREEN COMPOSITION Publication Classification (71) Applicants: James E. Hanson, Chester, NJ (US); (51) Int. Cl. Cosimo Antonacci, East Hanover, NJ A6R8/97 (2006.01) (US) A61O 1704 (2006.01) (52) U.S. Cl. (72) Inventors: James E. Hanson, Chester, NJ (US); CPC. A61K 8/97 (2013.01); A61O 1704 (2013.01) Cosimo Antonacci, East Hanover, NJ USPC ............................................... 424/60; 424/59 (US) (57) ABSTRACT A composition for Sunscreen or Sunscreen enhancer is dis (21) Appl. No.: 13/795,305 closed. The composition includes UV-blocking component comprising natural extracts, natural oils or nutrients or a (22) Filed: Mar 12, 2013 combination of these. The composition is capable of protect ing skin from the harmful effects of UV-light and it is capable Related U.S. Application Data of acting as an enhancer of Sunscreen actives, such as Zinc (60) Provisional application No. 61/685, 166, filed on Mar. oxide, titanium dioxide or other Sunscreen actives, such as 13, 2012, provisional application No. 61/685,460, Avobenzone, Dioxybenzone, Ecamsule, Meradimate, Oxy filed on Mar. 19, 2012, provisional application No. benZone, Sulisobenzone, Cinoxate, Ensulizole, Homosalate, 61/690,257, filed on Jun. 23, 2012, provisional appli Octinoxate, Octisalate, Octocrylene PABA, Padimate O or cation No. 61/690,280, filed on Jun. 23, 2012. Trolamine Salicylate. US 2013/0243709 A1 Sep. 19, 2013 NATURAL SUNSCREEN COMPOSITION 0006 To overcome these undesirable side effects of organic and inorganic sunscreen agents, there is a need for PRIORITY new formulations that can protect the skin from the harmful effects of ultraviolet radiation without any undesirable side 0001) This application claims priority of U.S. -

(Rosaceae), I. Differentiation of Mespilus and Crataegus
Phytotaxa 257 (3): 201–229 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2016 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.257.3.1 STUDIES IN MESPILUS, CRATAEGUS, AND ×CRATAEMESPILUS (ROSACEAE), I. DIFFERENTIATION OF MESPILUS AND CRATAEGUS, EXPANSION OF ×CRATAEMESPILUS, WITH SUPPLEMENTARY OBSERVATIONS ON DIFFERENCES BETWEEN THE CRATAEGUS AND AMELANCHIER CLADES JAMES B. PHIPPS Department of Biology, The University of Western Ontario, London, Ontario N6A 5B7, Canada; email: [email protected] Abstract The paper argues the position for retaining a monotypic Mespilus, i.e., in the sense of M. germanica, the medlar. Recent cladistic papers lend support for Mespilus being sister to Crataegus, and there is a clear morphological distinction from Cra- taegus, emphasized by adaptation to carnivore frugivory. Mespilus secured, the paper then treats each of the known hybrids between Mespilus and Crataegus, making the new combination Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps. Keywords: Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps comb. nov.; inflorescence position; medlar; Mespilus a folk-genus; Mespilus distinct from Crataegus; Rosaceae; taxonomic history of Mespilus Introduction The author has a long-standing interest in generic delimitation in the Maloid genera of the Rosaceae (Maleae Small, formerly Maloideae C. Weber, Pyrinae Dumort.), as shown particularly in a series of papers with K. Robertson, J. Rohrer, and P.G. Smith (Phipps et al. 1990, 1991; Robertson at al. 1991, 1992; Rohrer at al. 1991, 1994) which treated all 28 genera of Maleae as recognised by us. There is also a revisionary treatment of New World Heteromeles M.J.