Homologies of the Carpal Bones in Flying Squirrels (Pteromyinae): a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
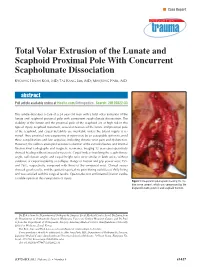
Total Volar Extrusion of the Lunate and Scaphoid Proximal Pole with Concurrent Scapholunate Dissociation
n Case Report Total Volar Extrusion of the Lunate and Scaphoid Proximal Pole With Concurrent Scapholunate Dissociation KYOUNG HWAN KOH, MD; TAE KANG LIM, MD; MIN JONG PARK, MD abstract Full article available online at Healio.com/Orthopedics. Search: 20120822-33 This article describes a case of a 24-year-old man with a total volar extrusion of the lunate and scaphoid proximal pole with concurrent scapholunate dissociation. The viability of the lunate and the proximal pole of the scaphoid are at high risk in this type of injury. Scaphoid nonunion, avascular necrosis of the lunate and proximal pole of the scaphoid, and carpal instability are inevitable unless the blood supply is re- stored. Thus, proximal row carpectomy at injury may be an acceptable option to avoid these complications and late sequelae, including chronic wrist pain and dysfunction. However, the authors attempted accurate reduction of the extruded bones and internal fixation.Final radiographs and magnetic resonance imaging 12 years postoperatively showed healing without avascular necrosis. Carpal indices involving the scapholunate angle, radiolunate angle, and carpal height ratio were similar in both wrists without evidence of carpal instability or collapse. Range of motion and grip power were 75% and 76%, respectively, compared with those of the uninjured wrist. Clinical scores showed good results, and the patient reported no pain during activities of daily living and was satisfied with his surgical results. Open reduction and internal fixation can be a viable option in this rare pattern of injury. Figure: Intraoperative photograph showing the me- dian nerve (arrow), which was compressed by the displaced lunate (asterisk) and scaphoid fracture. -

Fractures Long Bones, Upper Limb (Includes Hand)
Ministry of Defence Synopsis of Causation Fractures Long Bones, Upper Limb (includes hand) Author: Mr John A Dent, Ninewells Hospital and Medical School, Dundee Validator: Mr Sheo Tibrewal, Queen Elizabeth Hospital, London September 2008 Disclaimer This synopsis has been completed by medical practitioners. It is based on a literature search at the standard of a textbook of medicine and generalist review articles. It is not intended to be a meta-analysis of the literature on the condition specified. Every effort has been taken to ensure that the information contained in the synopsis is accurate and consistent with current knowledge and practice and to do this the synopsis has been subject to an external validation process by consultants in a relevant specialty nominated by the Royal Society of Medicine. The Ministry of Defence accepts full responsibility for the contents of this synopsis, and for any claims for loss, damage or injury arising from the use of this synopsis by the Ministry of Defence. 2 1. Definition 1.1. A bone breaks as a result of a variety of injuring forces. The fracture produced in a long bone may be of its shaft or diaphysis; towards one of its ends where the bone widens (metaphysis); or of the end of the bone, where it forms a joint with the next bone (Figure 1). Fractures near a joint may involve the joint surface causing intra-articular fractures. 1.2. It must always be remembered that the soft tissues surrounding the bone will also be damaged to a varying extent by the injuring force. -
What Is a Scaphoid Fracture?
Sussex Hand CONDITION Surgery Scaphoid Fractures CONDITION The scaphoid is one of the small What is a bones that make up your wrist. It is the commonest wrist bone Scaphoid Fracture? to break. Normal Wrist Xray Sometimes the diagnosis is delayed, Sometimes an operation is often by months or years. In this recommended. The exact situation xrays, MRI and CT scans approach will depend on the type might all be required to make a full of injury. Commonly a screw is Rest of the Thumb assessment of what is needed. placed down the centre of the metacarpal wrist bones scaphoid to keep the fragments (8 small bones lined up and still whilst the bone in total) The scaphoid What treatment is heals. This can sometimes be bone needed for a scaphoid done in a minimally invasive way fracture? with very small incisions (see Distal ulna This depends on a great many ‘Percutaneous screw fixation of Distal radius things. Some important factors are: Scaphoid Fractures’). Later on 1. How old is the injury? Fresh bigger operations might be A fracture of the scaphoid fractures have better healing necessary to re-align a bent potential scaphoid and put new bone into 2. Which part of the scaphoid is the old fracture (see ‘ORIF of broken? The nearer the fracture is Scaphoid Fractures +/-bone to end of the scaphoid next to the grafting’). radius (proximal pole) the more Very obvious chance there is of problems with What is the outcome fracture in the healing. This is to do with the blood following a scaphoid middle (waist) supply to the scaphoid bone which fracture? of the scaphoid is not good in the proximal pole. -

Isolated Trapezoid Fractures a Case Report with Compilation of the Literature
Bulletin of the NYU Hospital for Joint Diseases 2008;66(1):57-60 57 Isolated Trapezoid Fractures A Case Report with Compilation of the Literature Konrad I. Gruson, M.D., Kevin M. Kaplan, M.D., and Nader Paksima, D.O., M.P.H. Abstract as an axial load5,6 or bending stress7 transmitted indirectly Isolated fractures of the trapezoid bone have been rarely to the trapezoid through the second metacarpal. We present reported in the literature, the mechanism of injury being a case of an acute, isolated trapezoid fracture that resulted an axial or bending load transmitted through the second from direct trauma to the distal carpus and that was treated metacarpal. We report a case of an isolated, nondisplaced nonoperatively. Additionally, strategies for diagnosis and trapezoid fracture that was sustained by direct trauma treatment, as well as a synthesis of the published results and subsequently treated successfully in a short-arm cast. for both isolated and concomitant trapezoid fractures, are Diagnostic and treatment strategies for isolated fractures presented. of the trapezoid bone are reviewed as well as the results of operative and nonoperative treatment. Case Report A 25-year-old right-hand dominant male presented to the ractures of the carpus most commonly involve the emergency room (ER) complaining of isolated right-wrist scaphoid,1 with typical physical examination findings pain and swelling of 1 day’s duration. The patient stated Fof “snuffbox” tenderness. This presentation is fre- that a heavy metal door at work had closed onto the back quently the result of the patient falling onto an outstretched of his wrist causing an immediate onset of swelling and hand. -

Upper Extremity Injuries in Pediatric Athletes
Review Article Page 1 of 10 Upper extremity injuries in pediatric athletes Kristen M. Sochol, Daniel A. Charen, Jaehon Kim Department of Orthopedics at Mount Sinai Hospital, New York, NY, USA Contributions: (I) Conception and design: All authors; (II) Administrative support: All authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Kristen M. Sochol, MD. Department of Orthopedics at Mount Sinai Hospital, 5E 98th St, New York, NY 10029, USA. Email: [email protected]. Abstract: Upper extremity injuries in the pediatric patient are common, but are often more difficult to diagnose compared to their adult counterparts due to the gradual progression of cartilage ossification. Common pediatric upper extremity injuries include fractures and soft tissue trauma. Less prevalent injuries include sport specific overuse injuries. Fractures in the pediatric population are best described using the Salter-Harris classification, which has management and prognostic implications. Most pediatric upper extremity injuries can be managed with an initial trial of immobilization and early range of motion, followed by surgical intervention if necessary. Children have a robust healing response to bony and soft tissue injuries, and have good outcomes with appropriate management. Keywords: Pediatric athletes; upper extremity; Salter-Harris; overuse; injury Received: 14 February 2018; Accepted: 08 May 2018; Published: 15 May 2018. doi: 10.21037/aoj.2018.05.04 View this article at: http://dx.doi.org/10.21037/aoj.2018.05.04 Introduction joints are constrained by a network of ligaments that are primarily named after their attachment sites. -

Capitate Metastases in Adenocarcinoma Lung: a Rare
Case Report Capitate Metastases in Adenocarcinoma PROVISIONAL PDF Lung: A Rare Occurrence Jaspreet KAUR1, Renu MADAN1, Maneesh Kumar VIJAY2, Pramod Kumar JULKA1, Goura Kishore RATH1 Submitted: 21 May 2014 1 Department of Radiation Oncology, DR BRA Institute Rotary Cancer Accepted: 19 Nov 2014 Hospital, All India Institute of Medical Sciences, New Delhi 110029, India 2 Department of Pathology, All India Institute of Medical Sciences, New Delhi 110029, India Abstract Metastatic carcinoma is the most common malignancy of the bone. Metastases to the upper limbs of the skeleton are extremely uncommon, with only 10–15% occurring in this region. Metastases to the hand and wrist comprise about 0.15% of all hand tumours, and only 0.1% of all metastases. Carpal bone metastases are much rarer than those to the metacarpal and phalangeal bones. They usually masquerade as more common hand pathology such as arthritis or osteomyelitis. Given the bleak prognosis of carpal metastatic disease in lung cancer, treatment of a metastasis to the hand is usually palliative. Contrary to earlier beliefs, palliative radiotherapy plays a significant role in pain relief and improving hand mobility in patients diagnosed with metastatic disease of the hand. We report a case of adenocarcinoma of the lung with metastases to the capitate bone of the carpus treated with palliative radiotherapy. Keywords: carpal bone, metastases, lung cancer, palliative, radiotherapy Introduction Case report Metastatic carcinoma is the most common A 52-year-old male presented with fever, left- malignancy of the bone. The skeleton is the sided chest pain and pain in the right wrist for two third most common site of metastases after months. -

Upper Limb Fractures in Rugby in Huddersfield 1986- 1 990
Br J Sp Med 1991; 25(3) From the Clinic Br J Sports Med: first published as 10.1136/bjsm.25.3.139 on 1 September 1991. Downloaded from Upper limb fractures in rugby in Huddersfield 1986- 1 990 K.S. Eyres FRCS', A. Abdel-Salam FRCS2 and J. Cleary FRCS3 Research Registrar, Department of Human Metabolism and Clinical Biochemistry, University of Sheffield, Sheffield, UK 2 Orthopaedic Registrar, Huddersfield Royal Infirmary, Huddersfield, UK 3 Orthopaedic Consultant, Huddersfield Royal Infirmary, Huddersfield, UK Most injuries sustained by rugby players affect the soft Case 1 tissues, and fracture is relatively uncommon. Whereas the lower limb is most affected in footballers, the upper limb A 24-year-old full-back fractured his left clavicle in a tends to be injured in rugby players. Thirty consecutive scrummage in 1988 (Figure 1). Six months later, he fractures and ten dislocations affecting the upper limb, sustained a direct blow to the left shoulder. Radio- sustained by 35 rugby players, are reported. graphs showed a fracture to the greater tuberosity of the left humerus (Figure 2). He was treated with a Keywords: Rugby injury, hamate fracture, sports injury collar and cuff and was able to return to matchplay after 2 months. Nine months later he fell onto his left hand after a tackle. Radiographs showed a sagittal Most injuries sustained in rugby matches are to the fracture of the body of the hamate and a fracture of soft tissues. Fractures are relatively uncommon, the base of the fourth metacarpal (Figure 3). He was estimated to account for only 4% of injuries in adult treated conservatively with plaster immobilization, matches and for 5% of injuries in school matches'. -

REVIEW ARTICLE Osteoarthritis of the Wrist
REVIEW ARTICLE Osteoarthritis of the Wrist Krista E. Weiss, Craig M. Rodner, MD From Harvard College, Cambridge, MA and Department of Orthopaedic Surgery, University of Connecticut Health Center, Farmington, CT. Osteoarthritis of the wrist is one of the most common conditions encountered by hand surgeons. It may result from a nonunited or malunited fracture of the scaphoid or distal radius; disruption of the intercarpal, radiocarpal, radioulnar, or ulnocarpal ligaments; avascular necrosis of the carpus; or a developmental abnormality. Whatever the cause, subsequent abnormal joint loading produces a spectrum of symptoms, from mild swelling to considerable pain and limitations of motion as the involved joints degenerate. A meticulous clinical and radiographic evaluation is required so that the pain-generating articulation(s) can be identi- fied and eliminated. This article reviews common causes of wrist osteoarthritis and their surgical treatment alternatives. (J Hand Surg 2007;32A:725–746. Copyright © 2007 by the American Society for Surgery of the Hand.) Key words: Wrist, osteoarthritis, arthrodesis, carpectomy, SLAC. here are several different causes, both idio- of events is analogous to SLAC wrist and has pathic and traumatic, of wrist osteoarthritis. been termed scaphoid nonunion advanced collapse Untreated cases of idiopathic carpal avascular (SNAC). Wrist osteoarthritis can also occur second- T 1 2 necrosis, as in Kienböck’s or Preiser’s disease, may ary to an intra-articular fracture of the distal radius or result in radiocarpal arthritis. Congenital wrist abnor- ulna or from an extra-articular fracture resulting in malities, such as Madelung’s deformity,3,4 can lead malunion and abnormal joint loading. -

Osteoid Osteoma of the Trapezoid Bone
'-DIDUL)1DMG0D]KDU Case Report Osteoid Osteoma of the Trapezoid Bone Dawood Jafari MD1)DULG1DMG0D]KDU0'1 Abstract Osteoid osteoma is a benign, bone-forming tumor that rarely involves the carpal bones. We report a case of osteoid osteoma of the trap- H]RLGFDUSDOERQHZLWKH[WHQVLRQWRWKHDGMDFHQWVHFRQGPHWDFDUSDOERQH&KURQLFZULVWSDLQDQGORFDOWHQGHUQHVVZHUHWKHPDMRUFOLQLFDO signs and symptoms. In chronic wrist pain osteoid osteoma and the possibility of extension to the adjacent bones should be considered. Keywords: &DUSDOERQHPHWDFDUSDORVWHRLGRVWHRPDWUDSH]RLG Cite the article as: Jafari D, Najd Mazhar F. Osteoid Osteoma of the Trapezoid Bone. Arch Iran Med. 2012; 15(12): 777 – 779. Introduction RSV\WKURXJKDGRUVDODSSURDFK:HXVHGDVPDOOGULOOELWDQG¿QH osteotome to remove the involved area, which included the adjacent steoid osteoma is a benign bone tumor that rarely localizes articular surface of the trapezoid. The biopsy specimen had a highly to the carpal bones.1,2 Wrist pain usually is the main com- vascular reddish nidus embedded in normal bone (Figure 5). We no- O plaint and because it rarely involves the carpal bone, diag- ticed that the articular surface of the second metacarpal was eroded nosis is often delayed. It has been reported in the scaphoid and lu- and softened (Figure 6). Following curettage, we sent the specimen nate areas; however, the trapezoid is an exceedingly rare location from the base of the second metacarpal in a separate container for for osteoid osteoma. Bifocal involvement of adjacent carpal bones pathologic analysis. The results of the histologic examinations of has been reported previously but to the best of our knowledge ex- both biopsy specimens indicated osteoid osteoma (Figure 7). Since tension of osteoid osteoma through the joint to adjacent bone has we had only one nidus at the CT scan and involvement of both trap- not been mentioned in the literature. -

Radiohamate Impingement After Proximal Row Carpectomy ‘Radiohamate Impingement PRC’
Acta Orthop. Belg., 2020, 86 e-supplement 1, 19-21 CASE REPORT Radiohamate impingement after proximal row carpectomy ‘Radiohamate impingement PRC’ Pieter Caekebeke, Luc De Smet From the Department of Orthopaedics UZ Leuven, Pellenberg, Belgium Radiocarpal impingement after PRC is a well-known from 0% to 18% (5). Radiocarpal and pisiform complication due to impingement of the radial styloid impingement have been described after PRC. The against the radial carpal bones. A less common first is probably due to the proximalization of the impingement syndrome is that of the pisiforme. distal row with impingement of the trapezium/ We describe a radiohamate impingement and its trapezoid against the radial styloid process. The diagnosis and treatment. Based on a case we saw treatment is a radial styloid process resection for at our practice. Diagnosis is bases on standard radiographs and SPECT-CT. The treatment is the first and a pisiformectomy for the latter (3,5). No initially conservative. Surgery is necessary when other impingement syndromes have been described. conservative treatment fails and consists of resectie We present a case of radiohamate impingement of the proximal pole of the hamate. syndrome after proximal row carpectomy. Keywords: Radiohamate ; impingement ; proximal row CASE REPORT carpectomy. A 53-year-old mechanic contacted us 1 year after a work-accident with localized radiocarpal INTRODUCTION pain and swelling. Radiographs showed a stage Proximal row carpectomy (PRC) is a well- two SLAC wrist. (Fig. 1) A PRC with synovectomy established motion-preserving salvage procedure was performed. The following 3 years were for degenerative disorders of the proximal carpal uneventful with no to minimal pain complaints. -

A Rare Combination of Two Uncommon Fractures of the Carpal Bones
12 Maart 1960 S.A. TYDSKRIF VIR GE 'EESKUNDE 221 whether a low tracheotomy would have produced better 5. There must be no evidence of any pulmonary com figures. I performed a low tracheotomy on the first bulbo plication indicating broncbial obstruction and atelectasis. spinal case to be treated by Lassen's method in the 1956 The difficulty in detubation which ometimes arises in Cape Town polio epidemic and I must confess the tendency infants \ ho have had a tracheotomy for any length of time of the tube to' slip down the right bronchus required constant is a familiar problem. In the first in tance a laryngo opyand watching. The right-angled tube designed by Crampton bronchoscopy hould be carried out to a certain whether Smith eliminates this hazard, however, and with it I think there is any tenosis or whether granulations are present in this objection to a below-isthmus tracheotomy falls away. the trachea. When such granulations have been encountered If tracheotomy is being performed with a view to inter they have, in my experience, in ariably been found to be mittent-positive-pressure respiration it is necessary to excise a prolapsing into the trachea from the margins of the toma window from the anterior tracheal wall. This should be oval and not arising within the trachea itself. I have found that the in shape with its greatest diameter in the axis of the trachea. most effective way of dealing with these is to remove them If the tracheotomy is for an obstructive lesion or for tracheo under endotracheal anaesthesia with aHartman's granulation-. -

Osteoblastoma of the Trapezoid Bone and Triquetral Bone: Report of Two Cases
CASE REPORT Acta Orthop Traumatol Turc 2013;47(5):376-378 doi:10.3944/AOTT.2013.3081 Osteoblastoma of the trapezoid bone and triquetral bone: report of two cases ‹brahim KAYA1, Burak BOYNUK2, Caner GÜNERBÜYÜK3, Ak›n U⁄RAfi4 1Department of Orthopedics and Traumatology, Haseki Training and Research Hospital, ‹stanbul, Turkey; 2Department of Orthopedics and Traumatology, Bak›rköy Ac›badem Hospital, ‹stanbul, Turkey; 3Department of Orthopedics and Traumatology, 29 May›s Hospital, ‹stanbul, Turkey; 4Department of Orthopedics and Traumatology, ‹stanbul Medipol University, School of Medicine, ‹stanbul, Turkey Osteoblastoma is a benign local aggressive tumor mostly localized in the vertebra or long bones. Carpal location and recurrence are extremely rare. Treatment options include either curettage or wide en bloc resection which causes functional disability in the hand and wrist and should be reserved only for recurrence. We present a case of recurrent trapezoid osteoblastoma previously treated with curet- tage of the trapezoid bone and a case of primary triquetral osteoblastoma. Key words: Curettage; osteoblastoma; trapezoid bone; triquetral bone. Osteoblastoma is a benign primary bone tumor first pain increased at night and had a good response to non- described as “giant osteoid osteoma” by Dahlin and steroidal analgesic drugs. Radiographs, computerized Johnson in 1954.[1] Later, in 1956, Lichtenstein and Jaffe tomography and magnetic resonance imaging (MRI) named this tumor “osteoblastoma” in two different arti- revealed findings resembling avascular necrosis of the cles.[2] It is an uncommon benign but locally aggressive trapezoid bone, periosteal reaction at the second tumor, most commonly located in the vertebral column metacarpal and generalized edema in the dorsal com- or metaphysis of long bones.