COVID-19 Vaccination Updated Sputnik V Vaccine Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
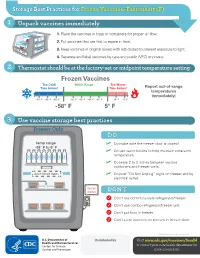
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

COVID-19 Vaccine Storage & Handling
COVID-19 Vaccine Storage & Handling Brooke Zeringue, Adrienne Whitney, & Robert Starszak Louisiana Department of Health Office of Public Health Immunization Program • Everyone (16 years and older) can be vaccinated. Louisiana Order COVID-19 vaccine in LINKS. Second COVID-19 doses are not automatically ordered. You must Vaccination order every dose you need. Guidelines All vaccine doses administered must be entered into LINKS within 24 hours and inputted as administered, NOT historical. COVID-19 Vaccine Delivery Storage & Handling for Moderna COVID-19 Vaccine: Direct from McKesson Vaccine will arrive frozen Larger quantities Thermal Shipper Storage & Handling for Pfizer- BioNTech COVID-19 Vaccine: Direct from Shipped in thermal shipping container Pfizer Vaccine arrives frozen Larger quantities Storage & Handling for Moderna COVID-19 Vaccine: Morris & Dickson Vaccine will arrive thawed Smaller Quantities Johnson & Johnson (Janssen) COVID-19 Vaccine Vaccine Type: • Viral Vector Vaccine Age indication: Overview of • 18 years or older. the Johnson & Dose & Route: Johnson • 0.5 mL; intramuscular COVID-19 Schedule: Vaccine • Single dose Dose Preparation: • 5 doses per vial Johnson & Johnson | STORING VACCINE VIALS Refrigerator 2°C to 8°C (36°F to 46°F) • Store up to the expiration date • DO NOT store frozen • Protect from light • You can find the expiration date at vaxcheck.jnj Johnson & Johnson | ADMINISTERING • Keep refrigerated until thawed (if arrived frozen). Refrigerator • PUNCTURED VIALS: Viable up to 6 hours 2°C to 8°C (36°F to 46°F) • If arrived frozen and needed immediately, thaw for Room 1 to 2 hours. • UNPUNCTURED VIALS: Viable up to 12 hours kept Temperature at 9°C to 25°C (47°F to 77°F) • PUNCTURED VIALS: Viable up to 2 hours Up to 25°C (Up to 77°F) Johnson & Johnson | ADMINISTERING Visually inspect the Before withdrawing each Johnson & Johnson COVID- dose of vaccine, carefully 19 vaccine vials for other mix the contents by Each dose must contain 0.5 particulate matter and/or swirling gently in an upright mL of vaccine. -

June 2021 2019
CURRENT AFFAIRS ORGANIC AND ORGANISED DECEMBERJUNE 2021 2019 A LETTER FROM MY HEART Dear IAS Aspirant Friends, It gives me immense pleasure to present to you the 360º Current Affairs Magazine for the month of June 2021. The dedicated team that compiles and edits Current Affairs at IAS WINNISHERS has made sincere efforts to provide to you the most relevant and important news from the point of view of Interview, Mains and especially the soon approaching Prelims. Our mission is to build IAS aspirants into human beings who can become IAS officers. In that direction, we strive to facilitate the current affairs knowledge that is ORGANIC and ORGANISED. Due to the ongoing unfortunate situation, we fully empathize with your anxiety related to the exam. This compilation aids you in your preparation, especially the soon approaching Prelims exam. This issue also carries information on INTERVIEW GUIDANCE PROGRAM conducted by IAS WINNISHERS, which has produced amazing results in the past. Get more information on our website and benefit immensely from it. Wishing You Success Vinay Kumar R Founder & CEO, IAS WINNISHERS Vinay Kumar R International NLP & IAS Coach 9036113902 | 9886273325 www.iaswinnishers.com © Winnishers Educational Services Pvt Ltd © Winnishers Educational Services Pvt Ltd 1 Contents 1. POLITY & CONSTITUTION ............................................................................................................ 8 1.1.LAST ‘D-VOTER’ WALKS OUT OF ASSAM DETENTION CENTRE ................................................................ -

Dutch Statutory Board Report and Financial Statements of Curevac N.V
Dutch statutory board report and financial statements of CureVac N.V. for the fiscal year ended December 31, 2020 Table of Contents Dutch Statutory Board Report ......................................................................................................... 3 1. Introduction......................................................................................................................................... 3 1.1 Preparation ................................................................................................................................... 3 1.2 Forward-Looking Statements .............................................................................................. 3 2. Company and business overview .............................................................................................. 5 2.1 History and development of the Company .................................................................... 5 2.2 Business overview .................................................................................................................... 5 2.3 Organizational Structure ................................................................................................... 120 2.4 Property, Plant and Equipment....................................................................................... 120 2.5 Material subsequent events ............................................................................................. 123 3. Financial Overview ...................................................................................................................... -

2021 Vaccine Storage and Handling
2021 Vaccine Storage and Handling Maine Immunization Program Annual Education Requirement Vaccines for Children Learning Objectives Learning Objectives At the conclusion of this training, the participant will be able to: 1. Define and explain cold chain management. 2. Describe the components of routine and emergency procedures for vaccine storage and handling. 3. Describe the roles of the primary and backup coordinators and other staff in the storage and handling of vaccines. 4. Describe proper vaccine storage and temperature monitoring equipment. 5. Describe correct vaccine and diluent storage, handling, and disposal of routinely recommended vaccines. 6. Identify actions that should be taken if vaccines have not been stored properly. Maine Center for Disease Control and Prevention 2 Introduction Proper vaccine storage and handling has been an important factor in preventing and eradicating many common vaccine-preventable diseases. Yet, each year, storage and handling errors result in revaccination of many patients and significant financial loss due to wasted vaccine. Failure to store and handle vaccines properly can reduce vaccine potency, resulting in inadequate immune response in patients and poor protection against disease. Patients can lose confidence in vaccines and providers if they have to be revaccinated because the vaccines they have received may have been compromised. This annual education requirement provides an overview of approved vaccine storage and handling best practices. For more detailed information, refer to the CDC’s Vaccine Storage and Handling Toolkit. Maine Center for Disease Control and Prevention 3 Cold Chain The vaccine cold chain is a temperature-controlled environment used to maintain and distribute vaccines in optimal condition. -

National Deployment & Vaccination Plan
NATIONAL DEPLOYMENT & VACCINATION EPI PLAN (NDVP)FOR COVID-19 VACCINES 24th June 2021 (2021) Expanded Program on Immunization | Ministry of National Health Services Regulations & Coordination Islamabad 1 Table of Contents Introduction Country Profile Planning and Coordination Regulatory Preparedness Identification of Target Population Costing and Funding Vaccine Delivery Strategy Supply Chain Management Microplanning Human Resource Management and Training Recording and Reporting Healthcare Waste Management RCCE, Vaccine Acceptance and Uptake Monitoring Surveillance and AEFI Evaluation 2 List of Acronyms Acronym Full name ADB Asian Development Bank AEFI Adverse Event Following Immunization AJK Azad Jammu and Kashmir BAL Balochistan BHU Basic Health Unit CBV Community Based Volunteer CDA Capital Development Authority CEPI Coalition for Epidemic Preparedness Innovations CHWs Community Health Workers CMW Community Midwife COVID Coronavirus Disease CSOs Civil Society Organizations CVC COVID-19 Vaccination Counter CVT COVID-19 Vaccination Team CVIC COVID-19 Vaccine Introduction Costing Tool DHO District Health Officer DHQ District Headquarter DQA Data Quality Assessment DRAP Drug Regulatory Authority of Pakistan EMRO Eastern Mediterranean Regional Office EOC Emergency Operation Center EPI Expanded Program on Immunization FATA Federally Administered Tribal Areas FIC Fully Immunized Child GAVI The Vaccine Alliance GACVS Global Advisory Committee on Vaccine Safety GB Gilgit-Baltistan GF Gates Foundation HCP Health Care Provider ICC Inter-Agency -

COVID-19 Vaccination Plan MICHIGAN
COVID-19 Vaccination Plan MICHIGAN 10/16/2020 | VERSION 1.0 MICHIGAN COVID-19 VACCINATION PLAN COVID-19 Vaccination Planning for Michigan The Centers for Disease Control and Prevention (CDC) has asked the health departments in all states, including the Michigan Department of Health and Human Services (MDHHS), to submit a COVID-19 Vaccination Plan. The Interim Draft plan was submitted to CDC Friday, Oct. 16. It is important to note this plan will be modified and enhanced as we learn more details about the vaccines. There are still many unknowns so some of the details cannot yet be finalized. Michigan is waiting to learn when the vaccines will be made available; how much vaccine will be made available and how quickly sufficient quantities will be available for the general public; how the vaccine will be allocated to Michigan; what the storage and handling requirement will be on the vaccines; and what the priority groups will be for this vaccine. The plan lays out how we will operationalize the distribution of the COVID-19 vaccine in Michigan and how we will engage our vaccination partners to assure, over time, that we have the ability to protect all individuals who wish to receive the vaccine. It will be a phased approach based on the priority groups determined by the Advisory Committee for Immunization Practices at the Federal level. Since initial supplies of vaccines will be insufficient to meet the needs of the entire population, they will be prioritized. As more vaccine becomes available, vaccination efforts will be expanded until eventually all individuals in Michigan will have the opportunity to receive the vaccine. -

Moderna COVID-19 Vaccine Storage and Handling Summary
Moderna COVID-19 Vaccine Storage and Handling Summary Basics Store vaccine in a freezer or refrigerator. See guidance below for each storage unit. Use vaccine vials stored in the refrigerator before removing vials from frozen storage. Check and record storage unit temperature each workday. See guidance below for each type of temperature monitoring device. Save storage records for 3 years, unless your jurisdiction requires a longer time period. Deliveries Vaccine 1. The vaccine will arrive frozen between -50°C and -15°C (-58°F and 5°F). 2. Examine the shipment for signs of damage. 3. Open the box and remove TagAlert Temperature Monitor from box (placed in the inner box next to vaccine). 4. Check the TagAlert temperature monitoring device by pressing the blue “start and stop” button. Left arrow points to a green checkmark: The vaccine is ready to use. Store the vaccine at proper temperatures immediately. Right arrow points to a red X: The numbers 1 and/or 2 will appear in the display. Store the vaccine at proper temperatures and label DO NOT USE! Call the phone number indicated in the instructions or your jurisdiction's immunization program IMMEDIATELY! 5. The expiration date is NOT printed on the vaccine vial or carton. To determine the expiration date: Scan the QR code located on the outer carton, or Go to www.modernatx.com/covid19vaccine-eua/. Ancillary Supply Kit An ancillary kit with supplies will be provided for administering the vaccine. Administration supplies include needles, syringes, sterile alcohol prep pads, vaccination record cards (shot cards), and some PPE. -

COVID-19 Living Evidence Profile #1 (Version 3: 11 February 2021)
Appendices for COVID-19 Living Evidence Profile #1 (Version 3: 11 February 2021) Appendix 1: Methodological details We use a standard protocol for preparing living evidence profiles (LEP) to ensure that our approach to identifying research evidence as well as experiences from other countries and from Canadian provinces and territories are as systematic and transparent as possible in the time we were given to prepare the profile. Identifying research evidence For each LEP, we search our continually updated inventory of best evidence syntheses and guide to key COVID-19 evidence sources for: 1) guidelines developed using a robust process (e.g., GRADE); 2) full systematic reviews; 3) rapid reviews; 4) guidelines developed using some type of evidence synthesis and/or expert opinion; 5) protocols for reviews or rapid reviews that are underway; 6) titles/questions for reviews that are being planned; and 7) single studies (when no guidelines, systematic reviews or rapid reviews are identified). For the first version of this LEP, we also searched Health Systems Evidence (www.healthsystemsevidence.org) and HealthEvidence (www.healthevidence.org), to identify any relevant evidence documents that might have relevance to the COVID-19 vaccine roll-out, but were produced before the pandemic, given that the other sources searched were specific to COVID-19. In Health Systems Evidence, we searched for overviews of systematic reviews, systematic reviews of effects, systematic reviews addressing other questions, and protocols for systematic reviews, that may provide insights about vaccine-delivery systems by searching for ‘vaccine’ using the filters for ‘public health’ (under health-system sectors). In HealthEvidence, we searched using the categories for ‘Immunization’ and ‘Policy and Legislation’ under the intervention strategy filter combined with ‘Communicable Disease/Infection’ category under the topic filter. -

Vaccine Storage and Handling Toolkit-March 2021
Vaccine Storage and Handling Toolkit Updated with COVID-19 Vaccine Storage and Handling Information Addendum added September 29, 2021 September 2021 CS296544-B Table of Contents The Vaccine Storage and Handling Toolkit has been updated with an addendum to address proper storage, handling, transport, and emergency handling of COVID-19 vaccines. The addendum will be updated as new COVID-19 vaccine products are approved. Please check the CDC Vaccine Storage and Handling Toolkit website (www.cdc.gov/vaccines/hcp/admin/storage/toolkit/index.html) regularly for the most current version of the toolkit during the COVID-19 response. The addendum can be found on page 49. Introduction .........................................................................................................................................................................................................2 SECTION ONE: Vaccine Cold Chain ..............................................................................................................................................................4 SECTION TWO: Staff and Training .................................................................................................................................................................6 SECTION THREE: Vaccine Storage and Temperature Monitoring Equipment ......................................................................................8 SECTION FOUR: Vaccine Inventory Management ....................................................................................................................................16