CDC Vaccine Storage and Handling Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gavi's Vaccine Investment Strategy
Gavi’s Vaccine Investment Strategy Deepali Patel THIRD WHO CONSULTATION ON GLOBAL ACTION PLAN FOR INFLUENZA VACCINES (GAP III) Geneva, Switzerland, 15-16 November 2016 www.gavi.org Vaccine Investment Strategy (VIS) Evidence-based approach to identifying new vaccine priorities for Gavi support Strategic investment Conducted every 5 years decision-making (rather than first-come- first-serve) Transparent methodology Consultations and Predictability of Gavi independent expert advice programmes for long- term planning by Analytical review of governments, industry evidence and modelling and donors 2 VIS is aligned with Gavi’s strategic cycle and replenishment 2011-2015 Strategic 2016-2020 Strategic 2021-2025 period period 2008 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 RTS,S pilot funding decision VIS #1 VIS #2 VIS #3 MenA, YF mass campaigns, JE, HPV Cholera stockpile, Mid 2017 : vaccine ‘long list’ Rubella, Rabies/cholera studies, Oct 2017 : methodology Typhoid Malaria – deferred Jun 2018 : vaccine shortlist conjugate Dec 2018 : investment decisions 3 VIS process Develop Collect data Develop in-depth methodology and Apply decision investment decision framework for cases for framework with comparative shortlisted evaluation analysis vaccines criteria Phase I Narrow long list Phase II Recommend for Identify long list to higher priority Gavi Board of vaccines vaccines approval of selected vaccines Stakeholder consultations and independent expert review 4 Evaluation criteria (VIS #2 – 2013) Additional Health Implementation -

(ACIP) General Best Guidance for Immunization
8. Altered Immunocompetence Updates This section incorporates general content from the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host (1), to which CDC provided input in November 2011. The evidence supporting this guidance is based on expert opinion and arrived at by consensus. General Principles Altered immunocompetence, a term often used synonymously with immunosuppression, immunodeficiency, and immunocompromise, can be classified as primary or secondary. Primary immunodeficiencies generally are inherited and include conditions defined by an inherent absence or quantitative deficiency of cellular, humoral, or both components that provide immunity. Examples include congenital immunodeficiency diseases such as X- linked agammaglobulinemia, SCID, and chronic granulomatous disease. Secondary immunodeficiency is acquired and is defined by loss or qualitative deficiency in cellular or humoral immune components that occurs as a result of a disease process or its therapy. Examples of secondary immunodeficiency include HIV infection, hematopoietic malignancies, treatment with radiation, and treatment with immunosuppressive drugs. The degree to which immunosuppressive drugs cause clinically significant immunodeficiency generally is dose related and varies by drug. Primary and secondary immunodeficiencies might include a combination of deficits in both cellular and humoral immunity. Certain conditions like asplenia and chronic renal disease also can cause altered immunocompetence. Determination of altered immunocompetence is important to the vaccine provider because incidence or severity of some vaccine-preventable diseases is higher in persons with altered immunocompetence; therefore, certain vaccines (e.g., inactivated influenza vaccine, pneumococcal vaccines) are recommended specifically for persons with these diseases (2,3). Administration of live vaccines might need to be deferred until immune function has improved. -

Package Insert
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------------- CONTRAINDICATIONS ------------------------------------- These highlights do not include all the information needed to use ® • History of severe allergic reactions (e.g., anaphylaxis) to egg proteins, or any FLUVIRIN (Influenza Virus Vaccine) safely and effectively. See full ® ® component of FLUVIRIN , or life-threatening reactions to previous influenza prescribing information for FLUVIRIN . vaccinations. (4.1, 11) FLUVIRIN® (Influenza Virus Vaccine) Suspension for Intramuscular Injection ---------------------- WARNINGS AND PRECAUTIONS ------------------------------ 2017-2018 Formula • If Guillain-Barré syndrome has occurred within 6 weeks of receipt of prior Initial US Approval: 1988 influenza vaccine, the decision to give FLUVIRIN® should be based on -------------------------- INDICATIONS AND USAGE ---------------------------------- careful consideration of the potential benefits and risks. (5.1) ® • FLUVIRIN is an inactivated influenza virus vaccine indicated for active • Immunocompromised persons may have a reduced immune response to immunization of persons 4 years of age and older against influenza disease FLUVIRIN®. (5.2) caused by influenza virus subtypes A and type B contained in the vaccine (1). • The tip caps of the FLUVIRIN® prefilled syringes may contain natural rubber • FLUVIRIN® is not indicated for children less than 4 years of age because latex which may cause allergic reactions in latex sensitive individuals. there is evidence of diminished immune response -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
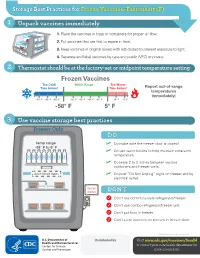
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

Global Challenges in Seasonal Influenza Vaccine Supply, Use, and Policy
Intersect, Vol 12, No. 1 (2018) Global Challenges in Seasonal Influenza Vaccine Supply, Use, and Policy Kaitlin Schroeder Stanford University Abstract Seasonal influenza outbreaks occur on every continent, infecting millions and killing around 500,000 patients each year. The 2018 influenza season has been a vicious one—the H3N2 strain is a fast-mutating, aggressive form of the virus, and we have had difficulty immunizing against it. Decreasing the devastation of seasonal influenza starts with smart vaccine systems: a greater supply of doses distributed to the right people. In this review, we evaluate the global threat of seasonal influenza and explore the availability of vaccines as an essential prevention mechanism. We first identify key stakeholders in global influenza policy, vaccine supply, and regional public health governance. Next, we define the extent of the need for vaccines by examining surveillance systems and assessing the current disease burden of seasonal influenza. We examine current challenges in vaccine availability and allocation, and their respective impacts on health outcomes. Finally, we discuss policy implications of supply and allocation studies; we particularly note the promising outlook of communication programs, the advantages of optimized dose distribution, and the need to concentrate on infrastructure in low-income countries. Schroeder, Global Influenza Vaccine Challenges Introduction “Vaccines are miracles.” Professor Pedro Alonso of the Institute of Global Health, Barcelona explains that just a few dollars spent on immunization can protect an individual from severe disease and disability, making vaccines “one of the best investments in health” (Robert, n.d.). Among the recognized vaccine-preventable diseases (VPDs) is seasonal influenza, an acute viral infection that affects millions of people worldwide every year. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Asplenia Vaccination Guide
Stanford Health Care Vaccination Subcommitee Revision date 11/308/2018 Functional or Anatomical Asplenia Vaccine Guide I. PURPOSE To outline appropriate vaccines targeting encapsulated bacteria for functionally or anatomically asplenic patients. Routine vaccines that may also be indicated but not addressed here include influenza, Tdap, herpes zoster, HPV, MMR, and varicella.1,2,3 II. Background Functionally or anatomically asplenic patients should be vaccinated to decrease the risk of sepsis due to organisms such as Streptococcus pneumoniae, Haemophilus influenzae type B, and Neisseria meningitidis. Guidelines are based on CDC recommendations. For additional information, see https://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html. III. Procedures/Guidelines1,2,3,6,7,8 The regimen consists of 4 vaccines initially, followed by repeat doses as specified: 1. Haemophilus b conjugate (Hib) vaccine (ACTHIB®) IM once if they have not previously received Hib vaccine 2. Pneumococcal conjugate 13-valent (PCV13) vaccine (PREVNAR 13®) IM once • 2nd dose: Pneumococcal polysaccharide 23-valent (PPSV23) vaccine (PNEUMOVAX 23®) SQ/IM once given ≥ 8 weeks later, then 3rd dose as PPSV23 > 5 years later.4 Note: The above is valid for those who have not received any pneumococcal vaccines previously, or those with unknown vaccination history. If already received prior doses of PPSV23: give PCV13 at least 1 year after last PPSV23 dose. 3. Meningococcal conjugate vaccine (MenACWY-CRM, MENVEO®) IM (repeat in ≥ 8 weeks, then every 5 years thereafter) 4. Meningococcal serogroup B vaccine (MenB, BEXSERO®) IM (repeat in ≥ 4 weeks) Timing of vaccination relative to splenectomy: 1. Should be given at least 14 days before splenectomy, if possible. -

Menhibrix® Meningococcal Groups C and Y and Haemophilus B Tetanus Toxoid Conjugate Vaccine
MenHibrix® Meningococcal Groups C and Y and Haemophilus b Tetanus Toxoid Conjugate Vaccine Age Indications for Menhibrix ® Vaccine Vaccine Administration Menhibrix® (GSK): for aged 6 weeks through 18 months at increased - Intramuscular (IM) injection in the anterolateral aspect of risk for meningococcal disease (see below) the thigh for most infants younger than 1 year of age In older children, in the deltoid muscle of the arm Indications for Use and Schedule - Indicated for active immunization to prevent invasive disease - 1-1.5 inch, 22-25 gauge needle - Use professional judgment in selecting caused by Neisseria meningitidis serogroups C and Y and needle length Haemophilus influenzae type b. Storage and Handling - Four doses (0.5 mL each) by intramuscular injection at 2, 4, 6, and 12 through 15 months of age. The first dose may be - Store in the refrigerator between given as early as 6 weeks of age. The fourth dose may be 35º-46º F (2º-8º C); Do NOT freeze Hib-MenCY-TT given as late as 18 months of age. - Protect vials from light. - Keep in the original box · Minimum interval - 8 weeks between doses - Administer vaccine immediately after drawn up in syringe MenHibrix will be supplied to select providers designated by the Georgia Immunization Office. HIGH- RISK INFANTS 6 WEEKS THROUGH 18 MONTHS OF AGE: Persons with persistent complement component pathway deficiencies Persons with anatomic or functional asplenia Complex congenital heart disease with asplenia Sickle cell disease Persons living in or traveling to countries in which N. meningitidis is hyper-endemic or epidemic. CONTRAINDICATIONS • An anaphylactic (severe allergic) reaction to a prior dose or a component of any meningococcal-, H. -

COVID-19 Vaccine Storage & Handling
COVID-19 Vaccine Storage & Handling Brooke Zeringue, Adrienne Whitney, & Robert Starszak Louisiana Department of Health Office of Public Health Immunization Program • Everyone (16 years and older) can be vaccinated. Louisiana Order COVID-19 vaccine in LINKS. Second COVID-19 doses are not automatically ordered. You must Vaccination order every dose you need. Guidelines All vaccine doses administered must be entered into LINKS within 24 hours and inputted as administered, NOT historical. COVID-19 Vaccine Delivery Storage & Handling for Moderna COVID-19 Vaccine: Direct from McKesson Vaccine will arrive frozen Larger quantities Thermal Shipper Storage & Handling for Pfizer- BioNTech COVID-19 Vaccine: Direct from Shipped in thermal shipping container Pfizer Vaccine arrives frozen Larger quantities Storage & Handling for Moderna COVID-19 Vaccine: Morris & Dickson Vaccine will arrive thawed Smaller Quantities Johnson & Johnson (Janssen) COVID-19 Vaccine Vaccine Type: • Viral Vector Vaccine Age indication: Overview of • 18 years or older. the Johnson & Dose & Route: Johnson • 0.5 mL; intramuscular COVID-19 Schedule: Vaccine • Single dose Dose Preparation: • 5 doses per vial Johnson & Johnson | STORING VACCINE VIALS Refrigerator 2°C to 8°C (36°F to 46°F) • Store up to the expiration date • DO NOT store frozen • Protect from light • You can find the expiration date at vaxcheck.jnj Johnson & Johnson | ADMINISTERING • Keep refrigerated until thawed (if arrived frozen). Refrigerator • PUNCTURED VIALS: Viable up to 6 hours 2°C to 8°C (36°F to 46°F) • If arrived frozen and needed immediately, thaw for Room 1 to 2 hours. • UNPUNCTURED VIALS: Viable up to 12 hours kept Temperature at 9°C to 25°C (47°F to 77°F) • PUNCTURED VIALS: Viable up to 2 hours Up to 25°C (Up to 77°F) Johnson & Johnson | ADMINISTERING Visually inspect the Before withdrawing each Johnson & Johnson COVID- dose of vaccine, carefully 19 vaccine vials for other mix the contents by Each dose must contain 0.5 particulate matter and/or swirling gently in an upright mL of vaccine. -

UNICEF Deep-Dive 2020, Vaccine Industry Consultation UNICEF’S Tentative Vaccine Tender Calendar for the Upcoming Period INFLUENZA Deep-Dive Agenda
UNICEF Deep-dive 2020, Vaccine Industry Consultation UNICEF’s Tentative Vaccine Tender Calendar for the upcoming period INFLUENZA Deep-dive Agenda • Background and challenges • Feedback from the industry • Gavi learning agenda for seasonal influenza • Tender timelines • Key takeaways 4 Background & Challenges • Immunization programmes, vaccines, and auxiliary supplies for seasonal influenza are primarily funded through a country’s own health budget. • Due to limited funding, seasonal influenza programmes are often not implemented in LICs and MICs. • Historically, UNICEF has procured limited quantities of influenza vaccine on behalf of countries. • UNICEF often receives requests for supply of seasonal influenza vaccine late and after suppliers have determined the production volume for the global market. • Due to COVID-19, the demand for seasonal influenza vaccines through UNICEF has increased during 2020, and realized late which let to constrained availability for countries procuring through UNICEF. UNICEF plans to tender earlier to counter this risk. Feedback from the industry What should UNICEF do differently to incentivize your participation in the future tenders for seasonal influenza vaccines? Feedback from the industry Measures taken by UNICEF • Tender one year in advance to block the • Advance tender timelines doses for UNICEF. • Stakeholders reminded of: • Multi-year tender and LTA o the short window for production planning and supply • UNICEF take risks and commit to pay for o importance of timely and accurate the doses even they are not materialised forecasting into orders. • Published a Supply Note on Seasonal Influenza Vaccines in English & French. • Working with countries to improve longer- term forecast accuracy. 6 Gavi learning agenda for seasonal influenza • Background: only 8% of Gavi eligible • Duration of the project: 2021 – 2022 countries have seasonal influenza immunization programmes, and most • Targeted countries: Cote d’Ivoire, Kenya & countries in the African region do not have Uganda national influenza immunization policies.