Moderna COVID-19 Vaccine Storage and Handling Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
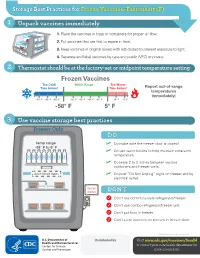
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

Interim Recommendations for Use of the Moderna Mrna-1273 Vaccine Against COVID-19
Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 Interim guidance First issued 25 January 2021 Updated 15 June 2021 Background This interim guidance has been developed on the basis of the advice issued by the Strategic Advisory Group of Experts on Immunization (SAGE) at its extraordinary meeting on 21 January 2021 (1) and updated during its extraordinary meeting on 27 May 2021(2). Declarations of interests were collected from all external contributors and assessed for any conflicts of interest. Summaries of the reported interests can be found on the SAGE meeting website and SAGE Working Group website. The guidance is based on the evidence summarised in the Background document on the Moderna mRNA-1273 vaccine against COVID-19 (3) and the background paper on COVID-19 disease and vaccines (4). Annexes which include GRADE and evidence-to-recommendations (ETR) tables have also been updated to reflect the updated recommendations. All referenced documents are available on the SAGE COVID-19 webpage: https://www.who.int/groups/strategic- advisory-group-of-experts-on-immunization/covid-19-materials. These interim recommendations refer to the mRNA-1273 vaccine, manufactured by Moderna. The vaccine is also known as COVID-19 Vaccine Moderna. In the subsequent text the vaccine will be referred to as mRNA-1273. On 30 April 2021, mRNA-1273 was granted WHO’s Emergency Use Listing (EUL). Methods SAGE applies the principles of evidence-based medicine and has set in place a thorough methodological process for issuing and updating recommendations (5). A detailed description of the methodological processes as they apply to COVID-19 vaccines can be found in the SAGE evidence framework for COVID-19 vaccines (6). -
Moderna Vaccine Day Presentation
Zika Encoded VLP mRNA(s) RSV CMV + EBV SARS-CoV- RSV-ped 2 Flu hMPV/PIV3 H7N9 Encoded mRNA(s) Ribosome Protein chain(s) First Vaccines Day April 14, 2020 © 2020 M oderna Therapeutics Forward-looking statements and disclaimers This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended including, but not limited to, statements concerning; the impact of the SARS-CoV-2 pandemic on the Company’s clinical trials and operations; the timing and finalization of a dose-confirmation Phase 2 study and planning for a pivotal Phase 3 study for mRNA-1647; the status and outcome of the Phase 1 clinical trial for mRNA-1273 being conducted by NIH; the next steps and ultimate commercial plan for mRNA-1273; the size of the potential market opportunity for mRNA-1273; the size of the potential commercial market for novel vaccines produced by Moderna or others; the potential peak sales for the Company’s wholly-owned vaccines; the probability of success of the Company’s vaccines individually and as a portfolio; and the ability of the Company to accelerate the research and development timeline for any individual product or the platform as a whole. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. -

The Covid–19 Pandemic and Haemoglobin Disorders
THE COVID–19 PANDEMIC AND HAEMOGLOBIN DISORDERS VACCINATIONS & THERAPEUTIC DRUGS An Informational Guide from the Thalassaemia International Federation (TIF) Prepared by: Dr Androulla Eleftheriou, Executive Director, TIF Last Updated: 12 May 2020 VACCINATIONS & THERAPEUTIC DRUGS Introduction It is important to note that there are currently no FDA1 or EMA2-approved or even recommended agents for the treatment of the novel coronavirus (COVID-19), for which the World Health Organization (WHO) declared as pandemic on Wednesday 11th of March 2020. Any agent being used at this time is being administered in an experimental setting under controlled conditions. Thalassaemia International Federation (TIF) has made an effort to compile a list of studies/clinical trials for treatment and vaccines, which is by no means exhaustive as this situation is extremely labile and research in this area is dramatically intensified. New information is anticipated to be added to this guide which is prepared exclusively for TIF’s global thalassemia community. The viral genome was mapped very soon as rom early January 2020 and shared globally. In February 2020, the WHO published an overview of the potential therapeutic candidates for the treatment of COVID-19. The document outlines 76 regimens that have been proposed (as of February 17, 2020) for the treatment of patients infected with the virus. Thirty-eight of these candidates are in the preclinical state with minimal information available on their proposed mechanism, uses, doses routes, or planned trials. Sixteen of the remaining regimens contain an interferon-based product. The rest include a variety of antimicrobials, corticosteroids, convalescent plasma, and biologics. The Director-General of the WHO, Mr Tedros Adhanom, stated on the 10th of April 2020, that more than 70 countries have joined WHO’s trial to accelerate research on effective treatments and 20 Institutions and companies ‘are racing to develop a vaccine’. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

Biopharma R&D on Treatment and Vaccines
Biopharma R&D on Treatment and Vaccines Coronavirus: Gilead Readies For Remdesivir Ramp Up, Questions On Profit Motive Demand Could Be High For Antiviral Executive Summary “All of that is at risk in not knowing if the drug If clinical trials prove it effective against COVID-19, works or not – and that is really not with an eye to remdesivir could potentially help millions of commercial,” she added. patients - which raises big questions about funding and access. Mercier said the company was spending more time on considering issues of access to the drug once its safety and efficacy is established – especially in regions of the world with less As vaccines will take around 12 months to developed healthcare systems. develop, companies with antiviral treatments are now been pushed to the front line in the battle Nevertheless, Gilead is contemplating a possible against coronavirus, and Gilead Sciences Inc.’s commercial future for the drug. remdesivir is currently the biggest hope of a drug to treat infected patients. “I have to be honest, commercial opportunity might come if this becomes a seasonal disease The company had big news to talk about on 2 or if stockpiling comes into play, but that is much March at the Cowen Health Care conference, as it later down the line,” concluded Mercier. had announced earlier in the day the acquisition of oncology firm Forty Seven for $4.9bn. (Also see One precedent for a big commercial hit for an “Gilead Calls Forty Seven Buyout Complementary antiviral product is Roche’s Tamiflu (oseltamivir). To Kite, Other IO Efforts” - Scrip, 2 Mar, 2020.) The drug achieved peak revenues of $3bn in 2009, largely down to stockpiling in response to that Speaking at the investor conference, Gilead’s chief year’s H1N1 flu pandemic. -

Effectiveness of Pfizer-Biontech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021
Morbidity and Mortality Weekly Report Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021 Mark W. Tenforde, MD, PhD1; Samantha M. Olson, MPH1; Wesley H. Self, MD2; H. Keipp Talbot, MD2; Christopher J. Lindsell, PhD2; Jay S. Steingrub, MD3; Nathan I. Shapiro, MD4; Adit A. Ginde, MD5; David J. Douin, MD5; Matthew E. Prekker, MD6; Samuel M. Brown, MD7; Ithan D. Peltan, MD7; Michelle N. Gong, MD8; Amira Mohamed, MD8; Akram Khan, MD9; Matthew C. Exline, MD10; D. Clark Files, MD11; Kevin W. Gibbs, MD11; William B. Stubblefield, MD2; Jonathan D. Casey, MD2; Todd W. Rice, MD2; Carlos G. Grijalva, MD2; David N. Hager, MD, PhD12; Arber Shehu, MD12; Nida Qadir, MD13; Steven Y. Chang, MD, PhD13; Jennifer G. Wilson, MD14; Manjusha Gaglani, MBBS15,16; Kempapura Murthy, MPH15; Nicole Calhoun, LMSW, MPA15; Arnold S. Monto, MD17; Emily T. Martin, PhD17; Anurag Malani, MD18; Richard K. Zimmerman, MD19; Fernanda P. Silveira, MD19; Donald B. Middleton, MD19; Yuwei Zhu, MD2; Dayna Wyatt2; Meagan Stephenson, MPH1; Adrienne Baughman2; Kelsey N. Womack, PhD2; Kimberly W. Hart2; Miwako Kobayashi, MD1; Jennifer R. Verani, MD1; Manish M. Patel, MD1; IVY Network; HAIVEN Investigators On April 28, 2021, this report was posted as an MMWR Early ≥65 years. Vaccination is a critical tool for reducing severe Release on the MMWR website (https://www.cdc.gov/mmwr). COVID-19 in groups at high risk. Adults aged ≥65 years are at increased risk for severe outcomes Randomized clinical trials of vaccines that have received an from COVID-19 and were identified as a priority group to EUA in the United States showed efficacy of 94%–95% in receive the first COVID-19 vaccines approved for use under preventing COVID-19–associated illness (4,5).§ However, an Emergency Use Authorization (EUA) in the United States hospitalization is a rare outcome among patients with (1–3). -

COVID-19 Vaccine Storage & Handling
COVID-19 Vaccine Storage & Handling Brooke Zeringue, Adrienne Whitney, & Robert Starszak Louisiana Department of Health Office of Public Health Immunization Program • Everyone (16 years and older) can be vaccinated. Louisiana Order COVID-19 vaccine in LINKS. Second COVID-19 doses are not automatically ordered. You must Vaccination order every dose you need. Guidelines All vaccine doses administered must be entered into LINKS within 24 hours and inputted as administered, NOT historical. COVID-19 Vaccine Delivery Storage & Handling for Moderna COVID-19 Vaccine: Direct from McKesson Vaccine will arrive frozen Larger quantities Thermal Shipper Storage & Handling for Pfizer- BioNTech COVID-19 Vaccine: Direct from Shipped in thermal shipping container Pfizer Vaccine arrives frozen Larger quantities Storage & Handling for Moderna COVID-19 Vaccine: Morris & Dickson Vaccine will arrive thawed Smaller Quantities Johnson & Johnson (Janssen) COVID-19 Vaccine Vaccine Type: • Viral Vector Vaccine Age indication: Overview of • 18 years or older. the Johnson & Dose & Route: Johnson • 0.5 mL; intramuscular COVID-19 Schedule: Vaccine • Single dose Dose Preparation: • 5 doses per vial Johnson & Johnson | STORING VACCINE VIALS Refrigerator 2°C to 8°C (36°F to 46°F) • Store up to the expiration date • DO NOT store frozen • Protect from light • You can find the expiration date at vaxcheck.jnj Johnson & Johnson | ADMINISTERING • Keep refrigerated until thawed (if arrived frozen). Refrigerator • PUNCTURED VIALS: Viable up to 6 hours 2°C to 8°C (36°F to 46°F) • If arrived frozen and needed immediately, thaw for Room 1 to 2 hours. • UNPUNCTURED VIALS: Viable up to 12 hours kept Temperature at 9°C to 25°C (47°F to 77°F) • PUNCTURED VIALS: Viable up to 2 hours Up to 25°C (Up to 77°F) Johnson & Johnson | ADMINISTERING Visually inspect the Before withdrawing each Johnson & Johnson COVID- dose of vaccine, carefully 19 vaccine vials for other mix the contents by Each dose must contain 0.5 particulate matter and/or swirling gently in an upright mL of vaccine. -

Comparing Three Covid-19 Vaccines: Pfizer, Moderna, J&J
Comparing three Covid-19 vaccines: Pfizer, Moderna, J&J 3/1/21, 2:09 PM Comparing the Covid-19 vaccines developed by Pfizer, Moderna, and Johnson & Johnson By Helen Branswell Feb. 2, 2021 CVS registered pharmacist Ken Ramey prepares to give a Covid-19 vaccine at the Isles of Vero Beach assisted and independent senior living community in Vero Beach, Fla. Wilfredo Lee/AP In an ideal world, a pandemic vaccine could be delivered in a single shot, so supplies could be stretched to cover a lot of people. It would trigger no side effect more significant than a sore arm. And it would be easy to ship and store. https://www.statnews.com/2021/02/02/comparing-the-covid-19-vaccines-developed-by-pfizer-moderna-and-johnson-johnson/ Page 1 of 10 Comparing three Covid-19 vaccines: Pfizer, Moderna, J&J 3/1/21, 2:09 PM We now have one such vaccine. On Feb. 27, the Food and Drug Administration announced it had issued an emergency use authorization for Johnson & Johnson’s one-dose Covid vaccine. Developed by J&J’s vaccines division, Janssen Pharmaceuticals, it was shown to be 66% protective against moderate to severe Covid infection in a multi-country study. Importantly, it was 85% effective in protecting against severe disease. And there were no hospitalizations or deaths among people in the vaccine arm of a large clinical trial. Overall efficacy varied a bit geographically, especially in South Africa, where a new variant appears to evade to some degree the immunity induced both by infection and by Covid vaccines, which were designed to target earlier strains of the SARS-CoV-2 virus. -

June 2021 2019
CURRENT AFFAIRS ORGANIC AND ORGANISED DECEMBERJUNE 2021 2019 A LETTER FROM MY HEART Dear IAS Aspirant Friends, It gives me immense pleasure to present to you the 360º Current Affairs Magazine for the month of June 2021. The dedicated team that compiles and edits Current Affairs at IAS WINNISHERS has made sincere efforts to provide to you the most relevant and important news from the point of view of Interview, Mains and especially the soon approaching Prelims. Our mission is to build IAS aspirants into human beings who can become IAS officers. In that direction, we strive to facilitate the current affairs knowledge that is ORGANIC and ORGANISED. Due to the ongoing unfortunate situation, we fully empathize with your anxiety related to the exam. This compilation aids you in your preparation, especially the soon approaching Prelims exam. This issue also carries information on INTERVIEW GUIDANCE PROGRAM conducted by IAS WINNISHERS, which has produced amazing results in the past. Get more information on our website and benefit immensely from it. Wishing You Success Vinay Kumar R Founder & CEO, IAS WINNISHERS Vinay Kumar R International NLP & IAS Coach 9036113902 | 9886273325 www.iaswinnishers.com © Winnishers Educational Services Pvt Ltd © Winnishers Educational Services Pvt Ltd 1 Contents 1. POLITY & CONSTITUTION ............................................................................................................ 8 1.1.LAST ‘D-VOTER’ WALKS OUT OF ASSAM DETENTION CENTRE ................................................................