Moderna Vaccine Day Presentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -

Print Layout 1
TECHNICAL PROGRAM Monday, March 19 5:00 – 8:00 pm Welcome Reception Tuesday, March 20 8:00 – 10:00 Session 1: Setting the Conference Context and Drivers Chair: Geoff Slaff (Amgen) Roger Perlmutter (Amgen) Conquering the Innovation Deficit in Drug Discovery Helen Winkle (CDER, FDA) Regulatory Modernization 10:00 – 10:30 Break Vendor and poster review 10:30 – 12:30 Session 2: Rapid Cell Line Development and Improved Expression System Development Chairs: Timothy Charlebois (Wyeth) and John Joly (Genentech) Amy Shen (Genentech) Stable Antibody Production Cell-Line Development with an Improved Selection Process and Accelerated Timeline Mark Leonard (Wyeth) High-Performing Cell-Line Development within a Rapid and Integrated Platform Process Control Pranhitha Reddy (Amgen) Applying Quality-by-Design to Cell Line Development Lin Zhang (Pfizer) Development of a Fully-Integrated Automated System for High-Throughput Screening and Selection of Single Cells Expressing Monoclonal Antibodies 12:30 – 2:00 Lunch Vendor and poster review 2:00 – 4:30 Session 3: High-Throughput Bulk Process Development Chairs: Brian Kelley (Wyeth) and Jorg Thommes (Biogen Idec) Colette Ranucci (Merck) Development of a Multi-Well Plate System for High-Throughput Process Development Min Zhang (SAFC Biosciences) CHO Media Library – an Efficient Platform for Rapid Development and Optimization of Cell Culture Media Supporting High Production of Pharmaceutical Proteins in Chinese Hamster Ovary Cells Nigel Titchener-Hooker The Use of Ultra-Scale-Down Approaches to Enable Rapid Investigation -

Interim Recommendations for Use of the Moderna Mrna-1273 Vaccine Against COVID-19
Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 Interim guidance First issued 25 January 2021 Updated 15 June 2021 Background This interim guidance has been developed on the basis of the advice issued by the Strategic Advisory Group of Experts on Immunization (SAGE) at its extraordinary meeting on 21 January 2021 (1) and updated during its extraordinary meeting on 27 May 2021(2). Declarations of interests were collected from all external contributors and assessed for any conflicts of interest. Summaries of the reported interests can be found on the SAGE meeting website and SAGE Working Group website. The guidance is based on the evidence summarised in the Background document on the Moderna mRNA-1273 vaccine against COVID-19 (3) and the background paper on COVID-19 disease and vaccines (4). Annexes which include GRADE and evidence-to-recommendations (ETR) tables have also been updated to reflect the updated recommendations. All referenced documents are available on the SAGE COVID-19 webpage: https://www.who.int/groups/strategic- advisory-group-of-experts-on-immunization/covid-19-materials. These interim recommendations refer to the mRNA-1273 vaccine, manufactured by Moderna. The vaccine is also known as COVID-19 Vaccine Moderna. In the subsequent text the vaccine will be referred to as mRNA-1273. On 30 April 2021, mRNA-1273 was granted WHO’s Emergency Use Listing (EUL). Methods SAGE applies the principles of evidence-based medicine and has set in place a thorough methodological process for issuing and updating recommendations (5). A detailed description of the methodological processes as they apply to COVID-19 vaccines can be found in the SAGE evidence framework for COVID-19 vaccines (6). -

The Covid–19 Pandemic and Haemoglobin Disorders
THE COVID–19 PANDEMIC AND HAEMOGLOBIN DISORDERS VACCINATIONS & THERAPEUTIC DRUGS An Informational Guide from the Thalassaemia International Federation (TIF) Prepared by: Dr Androulla Eleftheriou, Executive Director, TIF Last Updated: 12 May 2020 VACCINATIONS & THERAPEUTIC DRUGS Introduction It is important to note that there are currently no FDA1 or EMA2-approved or even recommended agents for the treatment of the novel coronavirus (COVID-19), for which the World Health Organization (WHO) declared as pandemic on Wednesday 11th of March 2020. Any agent being used at this time is being administered in an experimental setting under controlled conditions. Thalassaemia International Federation (TIF) has made an effort to compile a list of studies/clinical trials for treatment and vaccines, which is by no means exhaustive as this situation is extremely labile and research in this area is dramatically intensified. New information is anticipated to be added to this guide which is prepared exclusively for TIF’s global thalassemia community. The viral genome was mapped very soon as rom early January 2020 and shared globally. In February 2020, the WHO published an overview of the potential therapeutic candidates for the treatment of COVID-19. The document outlines 76 regimens that have been proposed (as of February 17, 2020) for the treatment of patients infected with the virus. Thirty-eight of these candidates are in the preclinical state with minimal information available on their proposed mechanism, uses, doses routes, or planned trials. Sixteen of the remaining regimens contain an interferon-based product. The rest include a variety of antimicrobials, corticosteroids, convalescent plasma, and biologics. The Director-General of the WHO, Mr Tedros Adhanom, stated on the 10th of April 2020, that more than 70 countries have joined WHO’s trial to accelerate research on effective treatments and 20 Institutions and companies ‘are racing to develop a vaccine’. -

Wyeth Pharmaceuticals Inc., a Subsidiary of Pfizer Inc Mylotarg
Mylotarg FDA ODAC Briefing Document 11 July 2017 WYETH PHARMACEUTICALS INC., A SUBSIDIARY OF PFIZER INC MYLOTARG (gemtuzumab ozogamicin; PF-05208747) In combination with chemotherapy for the treatment of previously untreated de novo CD33-positive acute myeloid leukemia and as monotherapy for the treatment of CD33-positive acute myeloid leukemia in first relapse FDA ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT (BLA 761060) Meeting Date: 11 July 2017 ADVISORY COMMITTEE BRIEFING MATERIALS: AVAILABLE FOR PUBLIC RELEASE 090177e18c1aa788\Approved\Approved On: 12-Jun-2017 13:05 (GMT) Page 1 Mylotarg FDA ODAC Briefing Document 11 July 2017 TABLE OF CONTENTS TABLE OF CONTENTS...........................................................................................................2 LIST OF TABLES.....................................................................................................................5 LIST OF FIGURES ...................................................................................................................6 1. EXECUTIVE SUMMARY .................................................................................................7 1.1. Introduction..............................................................................................................7 1.2. Rationale for Mylotarg Dosing Regimens ...............................................................8 1.3. Mylotarg in Patients With Previously Untreated De Novo AML............................9 1.4. Mylotarg in Patients With AML in First Relapse..................................................11 -

Biopharma R&D on Treatment and Vaccines
Biopharma R&D on Treatment and Vaccines Coronavirus: Gilead Readies For Remdesivir Ramp Up, Questions On Profit Motive Demand Could Be High For Antiviral Executive Summary “All of that is at risk in not knowing if the drug If clinical trials prove it effective against COVID-19, works or not – and that is really not with an eye to remdesivir could potentially help millions of commercial,” she added. patients - which raises big questions about funding and access. Mercier said the company was spending more time on considering issues of access to the drug once its safety and efficacy is established – especially in regions of the world with less As vaccines will take around 12 months to developed healthcare systems. develop, companies with antiviral treatments are now been pushed to the front line in the battle Nevertheless, Gilead is contemplating a possible against coronavirus, and Gilead Sciences Inc.’s commercial future for the drug. remdesivir is currently the biggest hope of a drug to treat infected patients. “I have to be honest, commercial opportunity might come if this becomes a seasonal disease The company had big news to talk about on 2 or if stockpiling comes into play, but that is much March at the Cowen Health Care conference, as it later down the line,” concluded Mercier. had announced earlier in the day the acquisition of oncology firm Forty Seven for $4.9bn. (Also see One precedent for a big commercial hit for an “Gilead Calls Forty Seven Buyout Complementary antiviral product is Roche’s Tamiflu (oseltamivir). To Kite, Other IO Efforts” - Scrip, 2 Mar, 2020.) The drug achieved peak revenues of $3bn in 2009, largely down to stockpiling in response to that Speaking at the investor conference, Gilead’s chief year’s H1N1 flu pandemic. -

Amgen and Wyeth Statement on FDA Announcement About Tumor Necrosis Factor (TNF) Blockers
Amgen and Wyeth Statement on FDA Announcement About Tumor Necrosis Factor (TNF) Blockers August 4, 2009 THOUSAND OAKS, Calif. and COLLEGEVILLE, Pa., Aug. 4 /PRNewswire-FirstCall/ -- Amgen (Nasdaq: AMGN) and Wyeth Pharmaceuticals, a division of Wyeth (NYSE: WYE), issued a statement in response to the Food and Drug Administration (FDA) announcement regarding the results of a safety review of Tumor Necrosis Factor (TNF) blockers [marketed as Remicade (infliximab), Enbrel (etanercept), Humira (adalimumab), Cimzia (certolizumab pegol) and Simponi (golimumab)]. This safety review was the subject of an FDA Early Communication in June 2008 pertaining to cases of malignancy in pediatric patients exposed to a TNF blocker. As a result of this review, the FDA has required strengthened warnings about the occurrence of lymphoma and other cancers in children and young adults using these medicines. AMGEN AND WYETH STATEMENT: Amgen and Wyeth believe that ENBREL continues to offer a favorable benefit-risk relationship for patients with the diseases for which it is indicated to treat, including moderate to severe Juvenile Idiopathic Arthritis (JIA). JIA can be a serious and potentially debilitating condition. Amgen will work with the FDA to update the U.S. Prescribing Information, and Medication Guide for ENBREL as described in the FDA communication which can be read at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm175803.htm. In addition, Amgen and Wyeth will communicate the revised product labeling to both physicians and patients. ENBREL was first approved for JIA in the U.S. in 1999. It is estimated through postmarketing data that approximately 13,847 pediatric patients have been treated with ENBREL globally through February 2009, accounting for approximately 44,600 patient-years of exposure. -

Effectiveness of Pfizer-Biontech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021
Morbidity and Mortality Weekly Report Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021 Mark W. Tenforde, MD, PhD1; Samantha M. Olson, MPH1; Wesley H. Self, MD2; H. Keipp Talbot, MD2; Christopher J. Lindsell, PhD2; Jay S. Steingrub, MD3; Nathan I. Shapiro, MD4; Adit A. Ginde, MD5; David J. Douin, MD5; Matthew E. Prekker, MD6; Samuel M. Brown, MD7; Ithan D. Peltan, MD7; Michelle N. Gong, MD8; Amira Mohamed, MD8; Akram Khan, MD9; Matthew C. Exline, MD10; D. Clark Files, MD11; Kevin W. Gibbs, MD11; William B. Stubblefield, MD2; Jonathan D. Casey, MD2; Todd W. Rice, MD2; Carlos G. Grijalva, MD2; David N. Hager, MD, PhD12; Arber Shehu, MD12; Nida Qadir, MD13; Steven Y. Chang, MD, PhD13; Jennifer G. Wilson, MD14; Manjusha Gaglani, MBBS15,16; Kempapura Murthy, MPH15; Nicole Calhoun, LMSW, MPA15; Arnold S. Monto, MD17; Emily T. Martin, PhD17; Anurag Malani, MD18; Richard K. Zimmerman, MD19; Fernanda P. Silveira, MD19; Donald B. Middleton, MD19; Yuwei Zhu, MD2; Dayna Wyatt2; Meagan Stephenson, MPH1; Adrienne Baughman2; Kelsey N. Womack, PhD2; Kimberly W. Hart2; Miwako Kobayashi, MD1; Jennifer R. Verani, MD1; Manish M. Patel, MD1; IVY Network; HAIVEN Investigators On April 28, 2021, this report was posted as an MMWR Early ≥65 years. Vaccination is a critical tool for reducing severe Release on the MMWR website (https://www.cdc.gov/mmwr). COVID-19 in groups at high risk. Adults aged ≥65 years are at increased risk for severe outcomes Randomized clinical trials of vaccines that have received an from COVID-19 and were identified as a priority group to EUA in the United States showed efficacy of 94%–95% in receive the first COVID-19 vaccines approved for use under preventing COVID-19–associated illness (4,5).§ However, an Emergency Use Authorization (EUA) in the United States hospitalization is a rare outcome among patients with (1–3). -
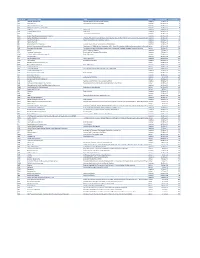
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

Comparing Three Covid-19 Vaccines: Pfizer, Moderna, J&J
Comparing three Covid-19 vaccines: Pfizer, Moderna, J&J 3/1/21, 2:09 PM Comparing the Covid-19 vaccines developed by Pfizer, Moderna, and Johnson & Johnson By Helen Branswell Feb. 2, 2021 CVS registered pharmacist Ken Ramey prepares to give a Covid-19 vaccine at the Isles of Vero Beach assisted and independent senior living community in Vero Beach, Fla. Wilfredo Lee/AP In an ideal world, a pandemic vaccine could be delivered in a single shot, so supplies could be stretched to cover a lot of people. It would trigger no side effect more significant than a sore arm. And it would be easy to ship and store. https://www.statnews.com/2021/02/02/comparing-the-covid-19-vaccines-developed-by-pfizer-moderna-and-johnson-johnson/ Page 1 of 10 Comparing three Covid-19 vaccines: Pfizer, Moderna, J&J 3/1/21, 2:09 PM We now have one such vaccine. On Feb. 27, the Food and Drug Administration announced it had issued an emergency use authorization for Johnson & Johnson’s one-dose Covid vaccine. Developed by J&J’s vaccines division, Janssen Pharmaceuticals, it was shown to be 66% protective against moderate to severe Covid infection in a multi-country study. Importantly, it was 85% effective in protecting against severe disease. And there were no hospitalizations or deaths among people in the vaccine arm of a large clinical trial. Overall efficacy varied a bit geographically, especially in South Africa, where a new variant appears to evade to some degree the immunity induced both by infection and by Covid vaccines, which were designed to target earlier strains of the SARS-CoV-2 virus. -
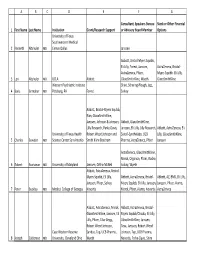
1 2 3 4 5 6 7 8 ABCDEFG First Name Last Name
ABC D E F G Consultant, Speakers Bureau Stock or Other Financial 1 First Name Last Name Institution Grant/Research Support or Advisory Board Member Options University of Texas Southwestern Medical 2 Kenneth Altshuler MD Center Dallas Janssen Abbott, Bristol‐Myers Squibb, Eli Lilly, Forest, Janssen, AstraZeneca, Bristol‐ AstraZeneca, Pfizer, Myers Squibb. Eli Lilly, 3 Lori Altshuler MD UCLA Abbott GlaxoSmithKline, Wyeth GlaxoSmithKline Western Psychiatric Institute Shire, Schering‐Plough, Jazz, 4 Boris Birmaher MD Pittsburg, PA Forest Solvay Abbott, Bristol‐Myers Squibb, Elan, GlaxoSmithKline, Janssen, Johnson & Johnson, Abbott, GlaxoSmithKline, Lilly Research, Parke‐Davis, Janssen, Eli Lilly, Lilly Research, Abbott, AstraZeneca, Eli University of Texas Health Robert Wood Johnson and Sanofi‐Synthelabo, UCB Lilly, GlaxoSmithKline, 5 Charles Bowden MD Science Center San Antonio Smith Kline Beecham Pharma, AstraZeneca, Pfizer Janssen AstraZeneca, GlaxoSmithKline, Merck, Organon, Pfizer, Roche, 6 Robert Buchanan MD University of Maryland Janssen, Ortho‐McNeil Solvay, Wyeth Abbott, AstraZeneca, Bristol‐ Myers Squibb, Eli Lilly, Abbott, AstraZeneca, Bristol‐ Abbott, AZ, BMS, Eli Lilly, Janssen, Pfizer, Solvay, Myers Squibb, Eli Lilly, Janssen, Janssen, Pfizer, Alamo, 7 Peter Buckley MD Medical College of Georgia Novartis Merck, Pfizer, Alamo, Novartis AstraZeneca Abbott, AstraZeneca, Merck, Abbott, AstraZeneca, Bristol‐ GlaxoSmithKline, Janssen, Eli Myers Squibb/Otsuka, Eli Lilly, Lilly, Pfizer, Ciba‐Geigy, GlaxoSmithKline, Janssen, Robert Wood