The Covid–19 Pandemic and Haemoglobin Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -

Abbvie Allergan Acquisition
Creating a New Diversified Biopharmaceutical Company The Combination of AbbVie and Allergan Investor Presentation June 25, 2019 NO OFFER OR SOLICITATION This presentation is not intended to and does not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities or the solicitation of any vote or approval in any jurisdiction pursuant to the acquisition or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. In particular, this presentation is not an offer of securities for sale into the United States. No offer of securities shall be made in the United States absent registration under the U.S. Securities Act of 1933, as amended, or pursuant to an exemption from, or in a transaction not subject to, such registration requirements. Any securities issued in the acquisition are anticipated to be issued in reliance upon available exemptions from such registration requirements pursuant to Section 3(a)(10) of the U.S. Securities Act of 1933, as amended. The acquisition will be made solely by means of the Scheme Document (or, if applicable, the Takeover Offer document), which will contain the full terms and conditions of the acquisition, including details with respect to the AbbVie shareholder vote in respect of the acquisition. Any decision in respect of, or other response to, the acquisition, should be made only on the basis of the information contained in the Scheme Document. IMPORTANT ADDITIONAL INFORMATION WILL BE FILED WITH THE SEC In connection with the proposed Acquisition, Allergan will file with the Securities Exchange Commission (the “SEC”) a Proxy Statement, which will include the Scheme Document. -

A Menace Wrapped in a Protein: Zika and the Global Health Security Agenda
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/320677119 Title: A MENACE WRAPPED IN A PROTEIN: ZIKA AND THE GLOBAL HEALTH SECURITY AGENDA Technical Report · September 2017 DOI: 10.13140/RG.2.2.25050.85443 CITATIONS READS 2 129 5 authors, including: Helen Epstein Wilmot James Bard College Columbia University 28 PUBLICATIONS 925 CITATIONS 5 PUBLICATIONS 15 CITATIONS SEE PROFILE SEE PROFILE Ángel G Muñoz Lawrence Stanberry Columbia University Columbia University 121 PUBLICATIONS 694 CITATIONS 238 PUBLICATIONS 7,326 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Climate informed malaria prevention, control and elimination View project ENACTS (Enhanced National Climate Services) View project All content following this page was uploaded by Ángel G Muñoz on 27 October 2017. The user has requested enhancement of the downloaded file. Title: A MENACE WRAPPED IN A PROTEIN: ZIKA AND THE GLOBAL HEALTH SECURITY AGENDA Authors: Helen Epstein, Wilmot James, Ángel G. Muñoz, Lawrence Stanberry & Madeleine C. Thomson. Reference: Columbia GHS&D Working Group Papers 2017-01 Date: September, 2017 Place: Columbia University Medical Center, Morgan Stanley Children’s Hospital, Office 102, 3959 Broadway CHC 1-102, New York City NY 10032, USA. Copyright: This work is licensed under the Creative Commons Attribution-NonCommercial- ShareAlike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/. A MENACE WRAPPED IN A PROTEIN1: ZIKA AND THE GLOBAL HEALTH SECURITY AGENDA Preface Stephen Nicholas & Marc Grodman… i 1.Zika: Implications for Global Health Security Helen Epstein… 1 2.Zika Pathogenesis, Diagnosis and Vaccines Lawrence Stanberry… 12 3.Climate Change, the Environment and the Zika Outbreak Madeleine C. -

The COVID-19 Infodemic* 07 08
01 Editorial 02 03 04 05 06 The COVID-19 Infodemic* 07 08 1 1 2 3 4 09 Sebastián García-Saisó, Myrna Marti, Ian Brooks, Walter H. Curioso, Diego González, 10 Victoria Malek,1 Felipe Mejía Medina,1 Carlene Radix,5 Daniel Otzoy,6 Soraya Zacarías,7 11 1 1 12 Eliane Pereira dos Santos and Marcelo D’Agostino 13 14 15 16 Suggested citation García-Saisó S, Marti M, Brooks I, Curioso WH, González D, Malek V, et al. The COVID-19 Infodemic. Rev Panam Salud Publica. 2021;45:e56. https://doi.org/10.26633/RPSP.2021.56 17 18 19 20 On 15 February 2020, during the Munich Security Conference The infodemic can negatively impact health and well-being 21 (1), the Director of the World Health Organization (WHO), Dr. and can also polarize public debate. Campaigns against public 22 Tedros Adhanom Ghebreyesus, stated that the fight against health measures, inaccurate or falsified epidemiological data, and 23 the COVID-19 pandemic was accompanied by a fight against fake or biased evidence can potentially alter people’s behavior. 24 an “infodemic”, leading to a series of initiatives by the WHO This puts extra pressure on health systems, as it undermines the 25 and other organizations to face this challenge. This situation is scope and efficiency of the various health intervention programs. 26 not new: others have occurred during other health emergen- The main factors contributing to the development of the info- 27 cies, but never one of the current magnitude, resulting from the demic are associated with a lack of digital literacy programs (5) 28 increased use of digital applications (2). -

Covid and That Other Pandemic—Lies T’S Been a Long Weekend
comment “NHS staff's TikTok dances are feeding the hostile social media beast” DAVID OLIVER “To establish trust, we need transparency and everyday language” HELEN SALISBURY PLUS Covid’s impact on defensive medicine; a child’s right not to be hit CRITICAL THINKING Matt Morgan Covid and that other pandemic—lies t’s been a long weekend. The growing number must also speak no evil. Instead, they scream it at of my colleagues self-isolating has led to the top of their lungs. Half of the profi les pushing the increasing gaps in our rota. Combined with the unethical, dangerous, and discredited case for herd unwelcome return of covid-19 to intensive care immunity through the Great Barrington Declaration Iunits throughout Europe, the long hard summer were artifi cial, bot-like accounts, amplifi ed by social is morphing into the longer, harder winter. I let out a media above the consensus view. slow exhale as I sink into my sofa after arriving home As doctors, nurses, and patients, we must move late on a Sunday night. Time to recharge, relax, and beyond blaming individuals who respond to these refl ect—but a million voices are shouting at me. bad ideas and bad incentives: instead, let us task A video on YouTube tells me that the covid tests I the companies with taking responsibility for their ordered were false. A Facebook post proclaims that output. If not, they can fund the intensive care masks are simply a tool of government oppression. beds, the staff sickness gaps, and the tissues Twitter advises me that those death certifi cates I’ve to wipe away tears when their broadcast written were lies. -

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

COVID-19 Speaker Key: MH Dr Margaret Harris TAG Dr Tedros
COVID-19 Speaker key: MH Dr Margaret Harris TAG Dr Tedros Adhanom Ghebreyesus TU Tu MR Dr Michael Ryan VA Vadia MK Dr Maria Van Kerkhove DI Diego IM Imogen CR Charles CO Corinne CS Costas MH Good morning, afternoon and evening. Welcome, everybody, and thank you for joining this latest WHO press briefing on COVID-19. As most of you are aware, we here at WHO headquarters in Geneva are practising physical distancing as one measure to stop COVID-19 transmission so from this week and onwards these press briefings will be held as virtual meetings with very few of us in the room. You can see we're well apart. Just some housekeeping; if you've joined online please use the raise your hand icon to indicate you want to ask a question. If you've joined by phone press # 9 on your keypad to ask a question. Listen out for your name. Sometimes names are not clear when we get them so my apologies in advance. We have here today three people who for most of you need no introduction. We have WHO director-general, Dr Tedros, our executive director of our emergency programme, Dr Mike Ryan, and our technical lead, Dr Maria Van Kerkhove. Dr Tedros will provide some opening remarks, then we'll open it for questions. I'd like to remind you we can only have one question per journalist. We've got limited time. This is a very busy time for everybody so please respect that. There are a lot of you and we want to give everybody a fair chance. -

COVID-19 Vaccination Strategy in China: a Case Study
Article COVID-19 Vaccination Strategy in China: A Case Study Marjan Mohamadi 1,†, Yuling Lin 1,*,† ,Mélissa Vuillet Soit Vulliet 1,†, Antoine Flahault 1, Liudmila Rozanova 1 and Guilhem Fabre 2 1 Institute of Global Health, University of Geneva, 1211 Geneva, Switzerland; [email protected] (M.M.); [email protected] (M.V.S.V.); antoine.fl[email protected] (A.F.); [email protected] (L.R.) 2 Department of Chinese, UFR 2, Université Paul Valéry Montpellier 3, 34199 Montpellier, France; [email protected] * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: The coronavirus disease 2019 (COVID-19) outbreak in China was first reported to the World Health Organization on 31 December 2019, after the first cases were officially identified around 8 December 2019. However, the case of an infected patient of 55 years old can probably be traced back on 17 November. The spreading has been rapid and heterogeneous. Economic, political and social impacts have not been long overdue. This paper, based on English, French and Chinese research in national and international databases, aims to study the COVID-19 situation in China through the management of the outbreak and the Chinese response to vaccination strategy. The coronavirus disease pandemic is under control in China through non-pharmaceutical interventions, and the mass vaccination program has been launched to further prevent the disease and progressed steadily with Citation: Mohamadi, M.; Lin, Y.; 483.34 million doses having been administered across the country by 21 May 2021. China is also Vulliet, M.V.S.; Flahault, A.; acting as an important player in the development and production of SARS-CoV-2 vaccines. -

NOV 2020 RR Updated CREAM.Indd
RESEARCH REPORTS Volume LXXXI November 2020 published by RESEARCH REPORTS AIER publishes over 100 articles per month that are distributed in digital form. Research Reports contains Business Conditions Monthly plus 12 of the most representative, chosen here for popularity, variety, and relevance. These articles are often reprinted in venues around the web, including Seeking Alpha, Intellectual Takeout, Mises Brasil, and dozens of other outlets. To read all of them, go to www.aier.org Business Conditions Monthly ROBERT HUGHES 1 The Year of Disguises ROGER W. KOOPS 9 AIER Hosts Top Epidemiologists, Authors of the Great Barrington Declaration AIER STAFF 20 The Great Barrington Declaration and Its Critics JENIN YOUNES 22 Reddit’s Censorship of The Great Barrington Declaration ETHAN YANG 25 The Sketchy Claims of the Case for a Mask Mandate PHILLIP W. MAGNESS 28 Lockdown: The New Totalitarianism JEFFREY A. TUCKER 32 What’s Behind The WHO’s Lockdown Mixed-Messaging STACEY RUDIN 35 Covid Is Not Categorically Different DONALD J. BOUDREAUX 41 The Devastating Economic Impact of Covid-19 Shutdowns AMELIA JANASKIE & PETER C. EARLE 43 Will Things Ever Go Back To Normal? JOAKIM BOOK 53 Does Anyone Trust the Fed? THOMAS L. HOGAN 56 QE Goes Global COLIN LLOYD 58 BUSINESS CONDITIONS MONTHLY Robert Hughes Senior Research Fellow AIER’s Leading Indicators Index Improves Again in October The U.S. economy continued to recover in October from the historic plunge in economic activity in the first half of the year. However, the pace of recovery in some areas has slowed. Furthermore, fallout from continuing restrictions (small business and personal bankruptcies) as well as the potential for renewed lockdown policies remain ongoing threats to future growth. -

Interim Recommendations for Use of the Moderna Mrna-1273 Vaccine Against COVID-19
Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 Interim guidance First issued 25 January 2021 Updated 15 June 2021 Background This interim guidance has been developed on the basis of the advice issued by the Strategic Advisory Group of Experts on Immunization (SAGE) at its extraordinary meeting on 21 January 2021 (1) and updated during its extraordinary meeting on 27 May 2021(2). Declarations of interests were collected from all external contributors and assessed for any conflicts of interest. Summaries of the reported interests can be found on the SAGE meeting website and SAGE Working Group website. The guidance is based on the evidence summarised in the Background document on the Moderna mRNA-1273 vaccine against COVID-19 (3) and the background paper on COVID-19 disease and vaccines (4). Annexes which include GRADE and evidence-to-recommendations (ETR) tables have also been updated to reflect the updated recommendations. All referenced documents are available on the SAGE COVID-19 webpage: https://www.who.int/groups/strategic- advisory-group-of-experts-on-immunization/covid-19-materials. These interim recommendations refer to the mRNA-1273 vaccine, manufactured by Moderna. The vaccine is also known as COVID-19 Vaccine Moderna. In the subsequent text the vaccine will be referred to as mRNA-1273. On 30 April 2021, mRNA-1273 was granted WHO’s Emergency Use Listing (EUL). Methods SAGE applies the principles of evidence-based medicine and has set in place a thorough methodological process for issuing and updating recommendations (5). A detailed description of the methodological processes as they apply to COVID-19 vaccines can be found in the SAGE evidence framework for COVID-19 vaccines (6). -
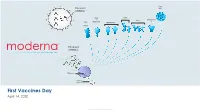
Moderna Vaccine Day Presentation
Zika Encoded VLP mRNA(s) RSV CMV + EBV SARS-CoV- RSV-ped 2 Flu hMPV/PIV3 H7N9 Encoded mRNA(s) Ribosome Protein chain(s) First Vaccines Day April 14, 2020 © 2020 M oderna Therapeutics Forward-looking statements and disclaimers This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended including, but not limited to, statements concerning; the impact of the SARS-CoV-2 pandemic on the Company’s clinical trials and operations; the timing and finalization of a dose-confirmation Phase 2 study and planning for a pivotal Phase 3 study for mRNA-1647; the status and outcome of the Phase 1 clinical trial for mRNA-1273 being conducted by NIH; the next steps and ultimate commercial plan for mRNA-1273; the size of the potential market opportunity for mRNA-1273; the size of the potential commercial market for novel vaccines produced by Moderna or others; the potential peak sales for the Company’s wholly-owned vaccines; the probability of success of the Company’s vaccines individually and as a portfolio; and the ability of the Company to accelerate the research and development timeline for any individual product or the platform as a whole. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. -

Biopharma R&D on Treatment and Vaccines
Biopharma R&D on Treatment and Vaccines Coronavirus: Gilead Readies For Remdesivir Ramp Up, Questions On Profit Motive Demand Could Be High For Antiviral Executive Summary “All of that is at risk in not knowing if the drug If clinical trials prove it effective against COVID-19, works or not – and that is really not with an eye to remdesivir could potentially help millions of commercial,” she added. patients - which raises big questions about funding and access. Mercier said the company was spending more time on considering issues of access to the drug once its safety and efficacy is established – especially in regions of the world with less As vaccines will take around 12 months to developed healthcare systems. develop, companies with antiviral treatments are now been pushed to the front line in the battle Nevertheless, Gilead is contemplating a possible against coronavirus, and Gilead Sciences Inc.’s commercial future for the drug. remdesivir is currently the biggest hope of a drug to treat infected patients. “I have to be honest, commercial opportunity might come if this becomes a seasonal disease The company had big news to talk about on 2 or if stockpiling comes into play, but that is much March at the Cowen Health Care conference, as it later down the line,” concluded Mercier. had announced earlier in the day the acquisition of oncology firm Forty Seven for $4.9bn. (Also see One precedent for a big commercial hit for an “Gilead Calls Forty Seven Buyout Complementary antiviral product is Roche’s Tamiflu (oseltamivir). To Kite, Other IO Efforts” - Scrip, 2 Mar, 2020.) The drug achieved peak revenues of $3bn in 2009, largely down to stockpiling in response to that Speaking at the investor conference, Gilead’s chief year’s H1N1 flu pandemic.