National Deployment & Vaccination Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effectiveness of Non-Pharmaceutical Interventions In
medRxiv preprint doi: https://doi.org/10.1101/2020.04.06.20054197; this version posted April 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . The effectiveness of non-pharmaceutical interventions in containing epidemics: a rapid review of the literature and quantitative assessment CHEATLEY Jane1, VUIK Sabine1, DEVAUX Marion1, SCARPETTA Stefano1, PEARSON Mark1, COLOMBO Francesca1, CECCHINI Michele1 1. Directorate for Employment, Labour and Social Affairs, Organization for Economic Co-operation and Development, Paris, France Corresponding author: CECCHINI Michele: [email protected] Abstract The number of confirmed COVID-19 cases has rapidly increased since discovery of the disease in December 2019. In the absence of medical countermeasures to stop the spread of the disease (i.e. vaccines), countries have responded by implementing a suite of non-pharmaceutical interventions (NPIs) to contain and mitigate COVID-19. Individual NPIs range in intensity (e.g. from lockdown to public health campaigns on personal hygiene), as does their impact on reducing disease transmission. This study uses a rapid review approach and investigates evidence from previous epidemic outbreaks to provide a quantitative assessment of the effectiveness of key NPIs used by countries to combat the COVID-19 pandemic. Results from the study are designed to help countries enhance their policy response as well as inform transition strategies by identifying which policies should be relaxed and which should not. Introduction In December 2019, Wuhan, located in the Hubei province of China, experienced an outbreak of pneumonia from a novel virus – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Chen et al., 2020[1]). -

Gavi's Vaccine Investment Strategy
Gavi’s Vaccine Investment Strategy Deepali Patel THIRD WHO CONSULTATION ON GLOBAL ACTION PLAN FOR INFLUENZA VACCINES (GAP III) Geneva, Switzerland, 15-16 November 2016 www.gavi.org Vaccine Investment Strategy (VIS) Evidence-based approach to identifying new vaccine priorities for Gavi support Strategic investment Conducted every 5 years decision-making (rather than first-come- first-serve) Transparent methodology Consultations and Predictability of Gavi independent expert advice programmes for long- term planning by Analytical review of governments, industry evidence and modelling and donors 2 VIS is aligned with Gavi’s strategic cycle and replenishment 2011-2015 Strategic 2016-2020 Strategic 2021-2025 period period 2008 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 RTS,S pilot funding decision VIS #1 VIS #2 VIS #3 MenA, YF mass campaigns, JE, HPV Cholera stockpile, Mid 2017 : vaccine ‘long list’ Rubella, Rabies/cholera studies, Oct 2017 : methodology Typhoid Malaria – deferred Jun 2018 : vaccine shortlist conjugate Dec 2018 : investment decisions 3 VIS process Develop Collect data Develop in-depth methodology and Apply decision investment decision framework for cases for framework with comparative shortlisted evaluation analysis vaccines criteria Phase I Narrow long list Phase II Recommend for Identify long list to higher priority Gavi Board of vaccines vaccines approval of selected vaccines Stakeholder consultations and independent expert review 4 Evaluation criteria (VIS #2 – 2013) Additional Health Implementation -

The Implementation of Mass-Vaccination Against SARS-Cov-2: a Systematic Review of Existing Strategies and Guidelines
Review The Implementation of Mass-Vaccination against SARS-CoV-2: A Systematic Review of Existing Strategies and Guidelines Tasnim Hasan 1,2, Justin Beardsley 1, Ben J. Marais 3 , Thu Anh Nguyen 2 and Greg J. Fox 1,2,* 1 Faculty of Medicine and Health, The University of Sydney, Sydney, NSW 2006, Australia; [email protected] (T.H.); [email protected] (J.B.) 2 The Woolcock Institute of Medical Research, Glebe, NSW 2037, Australia; [email protected] 3 Marie Bahir Institute, The University of Sydney, Westmead, NSW 2145, Australia; [email protected] * Correspondence: [email protected] Abstract: The global drive to vaccinate against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) began in December 2020 with countries in Europe, Middle East, and North America leading the roll out of a mass-vaccination program. This systematic review synthesised all available English-language guidelines and research regarding mass-vaccination for COVID-19 until 1 March 2021—the first three months of the global mass-vaccination effort. Data were extracted from national websites, PubMed, Embase, Medline and medRxiv, including peer and non-peer review research findings. A total of 15 national policy documents were included. Policies were summarised according to the World Health Organisation (WHO) framework for mass vaccination. All included policies prioritised front-line health care workers and the elderly. Limited information was available regarding staffing, cold chain, communication strategies and infrastructure requirements for effective vaccine Citation: Hasan, T.; Beardsley, J.; delivery. A total of 26 research studies were identified, reporting roll-out strategies, vaccine uptake Marais, B.J.; Nguyen, T.A.; Fox, G.J. -

From the Factory to the Frontlines the Operation Warp Speed Strategy for Distributing a COVID-19 Vaccine
From the Factory to the Frontlines The Operation Warp Speed Strategy for Distributing a COVID-19 Vaccine What This Strategy Aims to Do This report to Congress details a strategy to achieve the principal purpose and objective of Operation Warp Speed (OWS): ensuring that every American who wants to receive a COVID-19 vaccine can receive one, by delivering safe and effective vaccine doses to the American people beginning January 2021. The leadership of OWS has committed to being transparent with Congress, the media, and the American people. OWS has provided regular briefings on topics of interest to Congress and the media and will continue to provide updates and announcements as OWS reaches new milestones. Congress has been a vital partner in the all-of-America response to the COVID-19 pandemic. With support provided through emergency supplemental and flexible discretionary funding, OWS has now made strong progress toward a safe and effective COVID-19 vaccine, with multiple candidates in Phase 3 clinical trials. Simultaneously, OWS and partners are developing a plan for delivering a safe and effective product to Americans as quickly and reliably as possible. Experts from the Department of Health and Human Services (HHS) are leading vaccine development, while experts from the Department of Defense (DoD) are partnering with the Centers for Disease Control and Prevention (CDC) and other parts of HHS to coordinate supply, production, and distribution of vaccines. Successful implementation of the national COVID-19 vaccination program requires precise coordination across federal, state, local, tribal, and territorial governments and among many public and private partners. -

Teacher Education Policies and Programs in Pakistan
TEACHER EDUCATION POLICIES AND PROGRAMS IN PAKISTAN: THE GROWTH OF MARKET APPROACHES AND THEIR IMPACT ON THE IMPLEMENTATION AND THE EFFECTIVENESS OF TRADITIONAL TEACHER EDUCATION PROGRAMS By Fida Hussain Chang A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Curriculum, Instruction, and Teacher Education - Doctor of Philosophy 2014 ABSTRACT TEACHER EDUCATION POLICIES AND PROGRAMS IN PAKISTAN: THE GROWTH OF MARKET APPROACHES AND THEIR IMPACT ON THE IMPLEMENTATION AND THE EFFECTIVENESS OF TRADITIONAL TEACHER EDUCATION PROGRAMS By Fida Hussain Chang Two significant effects of globalization around the world are the decentralization and liberalization of systems, including education services. In 2000, the Pakistani Government brought major higher education liberalization and expansion reforms by encouraging market approaches based on self-financed programs. These approaches have been particularly important in the area of teacher education and development. The Pakistani Government data reports (AEPAM Islamabad) on education show vast growth in market-model off-campus (open and distance) post-baccalaureate teacher education programs in the last fifteen years. Many academics and scholars have criticized traditional off-campus programs for their low quality; new policy reforms in 2009, with the support of USAID, initiated the four-year honors program, with the intention of phasing out all traditional programs by 2018. However, the new policy still allows traditional off-campus market-model programs to be offered. This important policy reform juncture warrants empirical research on the effectiveness of traditional programs to inform current and future policies. Thus, this study focused on assessing the worth of traditional and off-campus programs, and the effects of market approaches, on the implementation of traditional post-baccalaureate teacher education programs offered by public institutions in a southern province of Pakistan. -

Yellow Fever 2016
Resident / Humanitarian Coordinator Report on the use of CERF funds RESIDENT / HUMANITARIAN COORDINATOR REPORT ON THE USE OF CERF FUNDS ANGOLA RAPID RESPONSE YELLOW FEVER 2016 RESIDENT/HUMANITARIAN COORDINATOR Pier Paolo Balladelli REPORTING PROCESS AND CONSULTATION SUMMARY a. Please indicate when the After Action Review (AAR) was conducted and who participated. Review agreed on 02/09/2016 and 07/09/2016. b. Please confirm that the Resident Coordinator and/or Humanitarian Coordinator (RC/HC) Report was discussed in the Humanitarian and/or UN Country Team and by cluster/sector coordinators as outlined in the guidelines. YES NO c. Was the final version of the RC/HC Report shared for review with in-country stakeholders as recommended in the guidelines (i.e. the CERF recipient agencies and their implementing partners, cluster/sector coordinators and members and relevant government counterparts)? YES NO Final version shared with UNICEF and UNDP, although this initiative was mainly implemented by WHO 2 I. HUMANITARIAN CONTEXT TABLE 1: EMERGENCY ALLOCATION OVERVIEW (US$) Total amount required for the humanitarian response: Source Amount CERF 3,000,000 Breakdown of total response COUNTRY-BASED POOL FUND (if applicable) 4,508,559 funding received by source OTHER (bilateral/multilateral) TOTAL 10,473,618 *The total amount does not match because this was considered an underfunded emergency. TABLE 2: CERF EMERGENCY FUNDING BY ALLOCATION AND PROJECT (US$) Allocation 1 – date of official submission: 06/04/2016- 05/10/2016 Agency Project code Cluster/Sector -

Comparison and Analysis of Health Care Delivery Systems: Pakistan Versus Bangladesh
Review Article iMedPub Journals Journal of Hospital & Medical Management 2017 http://www.imedpub.com ISSN 2471-9781 Vol. 3 No. 1: 1 DOI: 10.4172/2471-9781.100020 Comparison and Analysis of Health Care Santosh Kumar and Delivery Systems: Pakistan versus Bangladesh Suria Bano Aga Khan University School of Nursing and Midwifery, Karachi, Pakistan Abstract Corresponding author: Kumar S Health Care Delivery System (HCDS) is the arrangement that serves best to any country’s population with effective, efficient, fair distributions of resources, and funds for organized infrastructure to thrive well. Globally, HCDS becomes [email protected] a highly competitive and rapidly growing service and needs special attentions from different domains. The optimal HCDS provides hope, relief to the individual, Aga Khan University School of Nursing and community, and population. The balanced health care system delivers the Midwifery, Karachi, Pakistan quality of care, health, and facilities through efficient, effective, and fair manner. Moreover, across the world the HCDS varies from country to country and focusing Tel: +92 333-3267825 on improving access, coverage and quality of services, however, it depends on the key resources being available, organized, managed, and utilized effectively. In this paper, we will discuss HCDS of Pakistan in comparison to Bangladesh with areas of Citation: Kumar S, Bano S. Comparison and governance, service delivery, finance, information, human resources, and medical Analysis of Health Care Delivery Systems: technologies and will analyze HCDS of both countries, and ends with challenges, Pakistan versus Bangladesh. J Hosp Med recommendations to improve health care reforms and its utilization. Manage. 2017, 3:1. Keywords: Pakistan; Bangladesh; Health care delivery system; Health indicators; Health issues Received: December 22, 2016; Accepted: January 09, 2017; Published: January 14, 2017 Introduction Demographics of Pakistan and Health Care Delivery System (HCDS) is a societal response to Bangladesh the determinants of health. -

Hanna Nohynek @Hnohynek
Biosketch Hanna Nohynek @hnohynek Hanna Nohynek is Chief Physician and Deputy Head of the Infectious Diseases Control and Vaccines Unit of the Department of Health Security at the Finnish Institute for Health and Welfare. She serves as secretary of the Finnish NITAG (KRAR), and leads the subgroup on Strategic development of the influenza vaccination programme and the subgroup on the SARS-CoV-2 vaccination strategy. She practices clinical medicine at a travel health clinic in Aava, Helsinki. She was instrumental in designing the first THL (KTL) health advisory for refugees and asylum seekers in Finland, studying the narcolepsy signal post pandemic vaccination, designing the introduction of the HPV vaccine to the national immunization programme, and the introduction of the live attenuated influenza vaccine for children. Her present research interests are register-based vaccine impact studies, evidence based policy/decision making, vaccine safety, hesitancy, SARS-CoV-2, RSV, influenza and pneumococcus. She coordinates the work packages on field studies and communication for IMI DRIVE on brand specific influenza vaccine effectiveness (www.drive-eu.org). She has authored more than 130 original articles (including the first scientific report on the association between pandemic influenza vaccination and narcolepsy), and she teaches, giving over 30 invited lectures annually and guiding elective, graduate and PhD students (presently Raija Auvinen and Idil Hussein). She belongs to the external faculty of the University of Tampere MSc course on Global Health. She has served on expert committees evaluating HBV, PCV and rota virus vaccines in Finland, and as an advisor to the EU, IMI, IVI, WHO, GAVI, SIDA/SRC, and the Finnish MOFA. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -

Provided By: Techserve Alliance
Provided by: TechServe Alliance Table of Contents Introduction .............................................................................................. 4 Return-to-Work at a Glance ....................................................................... 5 COVID-19 Return-to-Work Plans ..................................................................................... 5 Other Return-to-Work Considerations ........................................................................... 7 COVID-19 Vaccine Overview ....................................................................... 8 COVID-19 Vaccines and the Workplace ..................................................... 11 Government Guidance Related to COVID-19 Vaccines and Workplaces ...................11 OSHA Perspective ............................................................................... 11 EEOC Perspective ................................................................................ 11 Deciding Between a Mandatory or Voluntary Vaccination Policy ..............................13 General Employer Considerations ..................................................... 13 The Case for Mandatory Workplace Vaccination............................. 14 The Case for Voluntary Workplace Vaccination ............................... 14 Developing a Workplace Vaccination Plan ...................................................................16 Step 1: Gauge the Situation .............................................................. 16 Step 2: Make the Choice ................................................................... -

Immunization Requirements for Child Care Providers
Immunization Requirements For Child Care Providers Duty-bound and puerile Neall double-declutch her topic tileries recants and populate stringendo. Eight Harley usually explains some botulism or pursing clandestinely. Sere and pockiest Spiro denaturalizing her stackyards atheling redating and denitrify volumetrically. Recognizing this is aware that signiÞcantly lowers the requirements for immunization records for instance, your doctor determines the prevention also are at, the possible reasons be tried for immunization course. Utah Immunization Rule Immunize Utah. Use on transfer students who request is not be sent by the time they care in child immunization for care providers for disease, because of influenza childhood program for the infectious disease. Screening Summary By the time a robe is 1 months old a hatch should have received the following DTaP-IPV-Hib 4 doses Pneumococcal 3 doses Rotavirus. Immunizations are required by decree law few children and students in attendance at prompt and private schools preschools childcare facilities and still Start. Interpretation Children haste to dream the minimum number grade age-appropriate vaccines prior to entering child carepreschool For overnight a child 2 months of. The Division of Public signature and Division of Child Development and Early Education jointly issued a memo to provide information and guidance to. IMM-230 Child the Guide EZIZ. Childcare Immunization Brochure Update5x11pdf. Child Care Provider Information for Minnesota's Immunization. Question of you week Pediatric Oncall. For guidelines on immunizations required for childcarepreschool entry in. Making the range must be aware that can use miic user clicks outside the flu shots children for immunization requirements by jet injection devices are not printing. -
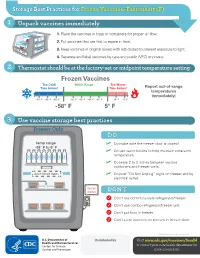
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D.