Pfizer-Biontech COVID-19 Vaccine Storage and Handling Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pfizer and Mylan Will Own 57% and 43% of the New Company (“Newco”), Respectively
DEAL PROFILE PFIZER | MYLAN VALUES 57% 43% $12.0bn PFIZER SHARE OF NEWCO MYLAN SHARE OF NEWCO NEW DEBT RAISED Pfizer Inc. (NYSE:PFE) Pfizer Inc. (“Pfizer”) is a pharmaceutical company that develops, manufactures, and sells products in internal medicine, vaccines, oncology, inflammation & immunology, and rare diseases. Upjohn, a subsidiary of Pfizer, is composed of Pfizer’s oral solid generics and off-patent drugs franchise, such as Viagra, Lipitor, and Xanax. Upjohn has a strong focus in emerging markets, and is headquartered in Shanghai, China. TEV: $265.4bn LTM EBITDA: $22.5bn LTM Revenue: $55.7bn Mylan N.V. (NASDAQ:MYL) Mylan N.V. (“Mylan”) is a pharmaceutical company that develops, manufactures, and commercializes generic, branded-generic, brand-name, and over-the-counter products. The company focuses in the following therapeutic areas: cardiovascular, CNS and anesthesia, dermatology, diabetes and metabolism, gastroenterology, immunology, infectious disease, oncology, respiratory and allergy, and women’s health. Mylan was founded in 1961 and is headquartered in Canonsburg, PA. TEV: $23.9bn LTM EBITDA: $3.5bn LTM Revenue: $11.3bn Bourne Partners provides strategic and financial advisory services to BOURNE clients throughout the business evolution life cycle. In order to provide PARTNERS the highest level of service, we routinely analyze relevant industry MARKET trends and transactions. These materials are available to our clients and partners and provide detailed insight into the pharma, pharma services, RESEARCH OTC, consumer health, and biotechnology sectors. On July 29, 2019, Pfizer announced the spin-off of its off-patent drug unit, Upjohn, in order to merge the unit with generic drug manufacturer, Mylan. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -

Third Quarter 2020
March 31, 2020 Third Quarter 2020 Corporate update and financial results November 10, 2020 Forward-looking statements Various statements in this slide presentation concerning the future expectations of BioNTech, its plans and prospects, including the Company's views with respect to the potential for mRNA and other pipeline therapeutics; BioNTech's efforts to combat COVID-19; the collaborations between BioNTech and Pfizer and Fosun to develop a potential COVID-19 vaccine; our expectations regarding the potential characteristics of BNT162b2 in our continuing Phase 2/3 trial and/or in commercial use based on data observations to date; the expected timepoint for additional readouts on efficacy data of BNT162b2 in our Phase 2/3 trial; the nature of the clinical data for BNT162, BNT311 and our other product candidates, which is subject to ongoing peer review, regulatory review and market interpretation; the timing for submission of data for, or receipt of, any potential approval or Emergency Use Authorization with respect to our BNT162 program; the timing for submission of BNT162 manufacturing data to the FDA; the ability of BioNTech to supply the quantities of BNT162 to support clinical development and, if approved, market demand, including our production estimates for 2020 and 2021 and orders received to-date; the planned next steps in BioNTech's pipeline programs and specifically including, but not limited to, statements regarding plans to initiate clinical trials of BioNTech's product candidates and expectations for data announcements with -
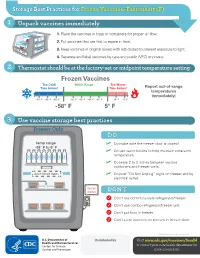
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

Companion Q&A Fact Sheet: What Mars Reveals About Life in Our
What Mars Reveals about Life in Our Universe Companion Q&A Fact Sheet Educators from the Smithsonian’s Air and Space and Natural History Museums assembled this collection of commonly asked questions about Mars to complement the Smithsonian Science How webinar broadcast on March 3, 2021, “What Mars Reveals about Life in our Universe.” Continue to explore Mars and your own curiosities with these facts and additional resources: • NASA: Mars Overview • NASA: Mars Robotic Missions • National Air and Space Museum on the Smithsonian Learning Lab: “Wondering About Astronomy Together” Guide • National Museum of Natural History: A collection of resources for teaching about Antarctic Meteorites and Mars 1 • Smithsonian Science How: “What Mars Reveals about Life in our Universe” with experts Cari Corrigan, L. Miché Aaron, and Mariah Baker (aired March 3, 2021) Mars Overview How long is Mars’ day? Mars takes 24 hours and 38 minutes to spin around once, so its day is very similar to Earth’s. How long is Mars’ year? Mars takes 687 days, almost two Earth years, to complete one orbit around the Sun. How far is Mars from Earth? The distance between Earth and Mars changes as both planets move around the Sun in their orbits. At its closest, Mars is just 34 million miles from the Earth; that’s about one third of Earth’s distance from the Sun. On the day of this program, March 3, 2021, Mars was about 135 million miles away, or four times its closest distance. How far is Mars from the Sun? Mars orbits an average of 141 million miles from the Sun, which is about one-and-a-half times as far as the Earth is from the Sun. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

Eople on the Move Eople on the Move
eopleeople onon PPthethe movemove Pierre Cloutier Sylvie Denis Gilles Fortin Nicolas Gauvin Bernard Michaud Anna Kratochvil Abbott Abbott Abbott Abbott Abbott Abbott Laboratories Laboratories Laboratories Laboratories Laboratories Laboratories Gary Schmid Alison Shore Eric Bergey Kal Dreisziger Lidia Krupka Abbott Abbott L’Académie- Allard-Johnson Allard-Johnson Laboratories Laboratories Ogilvy Communications Communications (Montreal) (Montreal) Pierre Cloutier, formerly Business Nicolas Gauvin, formerly Senior Alison Shore, formerly Product Unit Manager, Oncology, Product Manager, GI, has been Manager, has been promoted to Pharmaceutical Products Division, promoted to Marketing Manager, GI, Senior Product Manager, Metabolic has been promoted to Director, Pharmaceutical Products Division, at (Meridia), Pharmaceutical Products Customer Relations (Corporate), at Abbott Laboratories. Division, at Abbott Laboratories. Abbott Laboratories. Bernard Michaud, formerly Senior Eric Bergey, formerly International Sylvie Denis, formerly Business Unit Product Manager, Anti-infectives, has OTC Product Manager at World Manager, Cardiovascular & been promoted to Marketing Headquarters of Pharmacia & Metabolic, has been promoted to Manager, Anti-infectives, Upjohn in Sweden and, most Director of Sales, Primary Care, Pharmaceutical Products Division, at recently, Account Supervisor at Jeffrey Pharmaceutical Products Division, at Abbott Laboratories. Simbrow Associates (Montreal), has Abbott Laboratories. been appointed Account Director at Anna Kratochvil, -

Investor Presentation
Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Q1 2021 Results Investor presentation 1 Investor Relations │ Q1 2021 Results Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding the impact of the COVID-19 pandemic on certain therapeutic areas including dermatology, ophthalmology, our breast cancer portfolio, some newly launched brands and the Sandoz retail and anti-infectives business, and on drug development operations; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings of the Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions; or regarding the Group’s liquidity or cash flow positions and its ability to meet its ongoing financial obligations and operational needs; or regarding our collaboration with Molecular Partners to develop, manufacture and commercialize potential medicines for the prevention and treatment of COVID- 19 and our joining of the industry-wide efforts to meet global demand for COVID-19 vaccines and therapeutics by leveraging our manufacturing capacity and capabilities to support the production of the Pfizer-BioNTech vaccine and to manufacture the mRNA and bulk drug product for the vaccine candidate CVnCoV from CureVac. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

Johnson & Johnson and Pfizer Inc., Analysis of Agreement Containing
ANALYSIS OF AGREEMENT CONTAINING CONSENT ORDERS TO AID PUBLIC COMMENT In the Matter of Johnson & Johnson and Pfizer Inc. File No. 061-0220, Docket No. C-4180 I. Introduction The Federal Trade Commission (“Commission”) has accepted, subject to final approval, an Agreement Containing Consent Orders (“Consent Agreement”) from Johnson & Johnson (“J&J”) and Pfizer Inc. (“Pfizer”), which is designed to remedy the anticompetitive effects that would otherwise result from J&J’s proposed acquisition of Pfizer Consumer Healthcare. Under the terms of the proposed Consent Agreement, the parties will be required to divest: (1) Pfizer’s Zantac® H-2 blocker business; (2) Pfizer’s Cortizone® hydrocortisone anti-itch business; (3) Pfizer’s Unisom® nighttime sleep-aid business; and (4) J&J’s Balmex® diaper rash treatment business. The proposed Consent Agreement has been placed on the public record for thirty (30) days for receipt of comments by interested persons. Comments received during this period will become part of the public record. After thirty (30) days, the Commission will again review the proposed Consent Agreement and will decide whether it should withdraw from the proposed Consent Agreement, modify it, or make final the Decision and Order (“Order”). Pursuant to a Stock and Asset Purchase Agreement dated June 25, 2006, J&J proposes to acquire certain voting securities and assets comprising Pfizer’s Consumer Healthcare business in a transaction valued at approximately $16.6 billion (“Proposed Acquisition”). The Commission’s complaint alleges that the Proposed Acquisition, if consummated, would violate Section 7 of the Clayton Act, as amended, 15 U.S.C. -

Physical Changes
How Matter Changes By Cindy Grigg Changes in matter happen around you every day. Some changes make matter look different. Other changes make one kind of matter become another kind of matter. When you scrunch a sheet of paper up into a ball, it is still paper. It only changed shape. You can cut a large, rectangular piece of paper into many small triangles. It changed shape and size, but it is still paper. These kinds of changes are called physical changes. Physical changes are changes in the way matter looks. Changes in size and shape, like the changes in the cut pieces of paper, are physical changes. Physical changes are changes in the size, shape, state, or appearance of matter. Another kind of physical change happens when matter changes from one state to another state. When water freezes and makes ice, it is still water. It has only changed its state of matter from a liquid to a solid. It has changed its appearance and shape, but it is still water. You can change the ice back into water by letting it melt. Matter looks different when it changes states, but it stays the same kind of matter. Solids like ice can change into liquids. Heat speeds up the moving particles in ice. The particles move apart. Heat melts ice and changes it to liquid water. Metals can be changed from a solid to a liquid state also. Metals must be heated to a high temperature to melt. Melting is changing from a solid state to a liquid state.