Comfrey [72698-57-8] and One of Its Constituent Alkaloids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
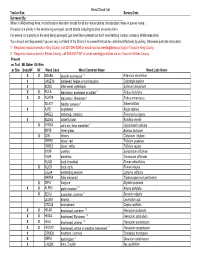
Weed Check List Survey Date
Weed Check List Trail or Site: Survey Date: Surveyed By: When in Wildnerness Area, record location and other details for all non-native plants. Include plant name in survey notes. If a weed is a priority in the area being surveyed, record details including location on survey form. For weeds not a priority in the area being surveyed, just mark them present but don't need lat/long location unless in Wilderness Area. You can pull and bag weeds if you are very confident of the ID and it is a weed that can be controlled effectively by pulling. Otherwise just note its location. 1 - Regulated noxious weeds in King County, call 206-296-0290 or email [email protected] if found in King County 2 - Regulated noxious weed in Kittitas County, call 509-962-7007 or email [email protected] if found in Kittitas County Present on Trail Mt. Baker Ok Wen or Site Snoq NF NF Weed Code Weed Common Name Weed Latin Name XXARAB3 absinth wormwood 1,2 Artemisia absinthium X CASE13 bindweed, hedge or morning glory Calystegia sepium X SODU bittersweet nightshade Solanum dulcamara XXRULA blackberry, evergreen or cutleaf 2 Rubus laciniatus XXRUAR9 blackberry, Himalayan 2 Rubus armeniacus SILA21 bladder campion 2 Silene latifolia X AJRE bugleweed Ajuga reptans RARE3 buttercup, creeping Ranunculus repens X BUDA2 butterfly bush Buddleia davidii X HYRA3 cat’s ear, false dandelion 2 Hypochaeris radicata BRTE cheat grass Bromus tectorum X CIIN chicory Cichorium intybus TRPR2 clover, red Trifolium pratense TRRE3 clover, white Trifolium repens SYOF comfrey Symphytum officinale TAOF dandelion Taraxacum officinale RUOB dock, broadleaf Rumex obtusifolius X RUCR dock, curly Rumex crispus LALA4 everlasting peavine Lathyrus latifolius MAPE2 false mayweed Tripleurospermum perforatum X DIPU foxglove Digitalis purpurea XXALPE4 garlic mustard 1,2 Alliaria petiolata X SEVU groundsel, common 2 Senecio vulgaris LEONT hawkbit Leontodon spp. -

Sterol Addition During Pollen Collection by Bees
Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies? Maryse Vanderplanck, Pierre-Laurent Zerck, Georges Lognay, Denis Michez To cite this version: Maryse Vanderplanck, Pierre-Laurent Zerck, Georges Lognay, Denis Michez. Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies?. Apidologie, 2020, 51 (5), pp.826-843. 10.1007/s13592-020-00764-3. hal-02784696 HAL Id: hal-02784696 https://hal.archives-ouvertes.fr/hal-02784696 Submitted on 3 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2020) 51:826–843 Original article * INRAE, DIB and Springer-Verlag France SAS, part of Springer Nature, 2020 DOI: 10.1007/s13592-020-00764-3 Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies? 1,2 1 3 1 Maryse VANDERPLANCK , Pierre-Laurent ZERCK , Georges LOGNAY , Denis MICHEZ 1Laboratory of Zoology, Research Institute for Biosciences, University of Mons, 20 Place du Parc, 7000, Mons, Belgium 2CNRS, UMR 8198 - Evo-Eco-Paleo, Univ. Lille, F-59000, Lille, France 3Analytical Chemistry, Agro Bio Chem Department, Gembloux Agro-Bio Tech University of Liège, 2 Passage des Déportés, 5030, Gembloux, Belgium Received 10 July 2019 – Revised2March2020– Accepted 30 March 2020 Abstract – Sterols are essential nutrients for bees which are thought to obtain them exclusively from pollen. -

Shoot Structure of Symphytum Officinale L. (Boraginaceae) in Relation to the Nature of Its Axially Shifted Lateral Branches
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Wulfenia Jahr/Year: 2011 Band/Volume: 18 Autor(en)/Author(s): Kotelnikova Ksenia V., Tretjakova Daria J., Nuraliev Maxim S. Artikel/Article: Shoot structure of Symphytum officinale L. (Boraginaceae) in relation to the nature of its axially shifted lateral branches. 63-79 © Landesmuseum für Kärnten; download www.landesmuseum.ktn.gv.at/wulfenia; www.biologiezentrum.at Wulfenia 18 (2011): 63 –79 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Shoot structure of Symphytum offi cinale L. (Boraginaceae) in relation to the nature of its axially shifted lateral branches Ksenia V. Kotelnikova, Daria J. Tretjakova & Maxim S. Nuraliev Summary: Shoot structure of Symphytum offi cinale was studied in terms of its architecture, vascular anatomy and morphogenesis. The main stem and lower stems of secondary order typically bear axially shifted lateral branches (along with axillary ones). This results in development of internode-like stem section between the lateral branch and its subtending leaf. Each shifted branch possesses two stem leaves (prophylls) and a terminal infl orescence. All lateral buds are initiated in the leaf axils. At later developmental stages some of them become shifted due to the intercalary growth of the stem between them and their subtending leaves. The intercalary stem portion possesses internodal stele structure, and the stele of the lateral stem branches from the main stele almost at the level of lateral stem insertion. This phenomenon can be treated as concaulescence (i.e. congenital fusion), though no strict evidences for phylogenetic fusion were found. Stems of Symphytum offi cinale also bear green wings of leafy nature. -

Boraginaceae), and the Phylogeny of Boraginoideae
!" #$ % " "& '()*"'+ (,-./01 ** -)2'/)*) %*()'-) %%*(* 3443 Dissertation for the Degree of Doctor of Philosophy in Systematic Botany presented at Uppsala University in 2002 Abstract Långström, E. 2002. Systematics of Echiochilon and Ogastemma (Boraginaceae), and the phylogeny of Boraginoideae. Acta Univ. Ups. Comprehensive Summaries og Uppsala Disserta- tions from the Faculty of Science and Technology 693. 34 pp. Uppsala. ISBN 91-554-5257-4. Echiochilon, Ogastemma and Sericostoma are revised resulting in the recognition of 15 spe- cies of Echiochilon and one Ogastemma species. Several species are placed in synonymy and three new species are described, E. baricum, E. callianthum and E. cyananthum. The single species of Sericostoma is shown to be nested within Echiochilon. The plastid atpB gene was sequenced for Echiochilon and Ogastemma from the Old World and Antiphytum from the New World, plus for a selection of 33 other Boraginaceae taxa. They were analysed together with selected outgroup taxa to give a framework of the tribes of Boraginoi- deae. The analysis gave support for establishing the new tribe Echiochileae for Antiphytum, Echio- chilon and Ogastemma, and for merging the traditionally accepted tribe Eritrichieae with Cyno- glosseae. The ITS region was sequenced for all but one species of Echiochilon and for representa- tives of Antiphytum and Ogastemma. Phylogenetic analysis of Echiochilon revealed that the strongly zygomorphic-flowered species form a paraphyletic group. The morphological data gave results fairly congruent with the ITS phylogeny. Biogeographic interpretations of the ITS and atpB phylogenies indicated a trans-Atlantic dispersal of Antiphytum as the most plausible explanation to the Old/New World disjunction. Analyses using DIVA (Dispersal Vicariance Analysis) of the distributions of the Echiochilon spe- cies indicated an ancestor to Echiochilon with a wide distribution over northern Africa and Arabia to India. -

Phylogeny of Eudicots (Or Tricolpates) Eudicots (Or Tricolpates) “Basal Eudicots”
Phylogeny of Eudicots (or Tricolpates) Eudicots (or Tricolpates) “Basal eudicots” Asterids Buxales Rosids Caryophyllales RanunculalesProteales After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374 Phylogeny of Asterids Asterids Lamiids Ericales Campanulids Cornales Cornales Ericales Campanulids: Lamiids: Aquifoliales Garryales Apiales Gentianales Dipsacales Lamiales Asterales Solanales After APG, 2003; Judd and Olmstead, 2004, and Soltis et al., 2005 Synapomorphies for Asterids and Core Asterids Synapomorphies for Asterids: Iridoid compounds; Unitegmic ovules; www.scielo.br/img/fbpe/jbchs/v12n2/a04fig01.gif Tenuinucellate ovules. Synapomorphies for Core Asterids (Lamiids + Campanulids): Gamopetalous corollas; A single whorl of stamens that alternate with the petal lobes; Epipetalous stamens; 2 fused carpels. Ericaceae (Rhododendron family) http://georgian.wunderground.com/data/wximagenew/f/Feather3/5431.jpg Rhododendron macrophyllum (Pacific Rhododendron, WA State Flower) Ericaceae (Rhododendron family) Textbook DVD WSJ Textbook DVD KRR & DLN Vaccinium corymbosum; The genus Vaccinium contains about 450 species, including blueberry, cranberry, huckleberry, etc.. Ericaceae (Rhododendron family) Photo: Yaowu Yuan Photo: Yaowu Yuan Gautheria shallon; Salal Cassiope tetragona http://www.nestlerode.org/ Cremation_Urn/urn_finished.jpg Photo: Yaowu Yuan Note the more or less pendulous Menziesia ferruginea flowers and the urn-shaped corolla. Ericaceae (Rhododendron family) Textbook DVD KRR & DLN Textbook DVD KMN Pieris japonica Vaccinium arboreum Note that the anthers often have spur- or awn-like appendages. Ericaceae (Rhododendron family) Photo: Yaowu Yuan Menziesia ferruginea Note that anther opens by 2 terminal pores –– poricidal dehiscence. Ericaceae (Rhododendron family) Textbook DVD KRR & DLN Kalmia latifolia Note that although corolla can be more spread and not urn-shaped, anther usually opens by terminal pores. -

Boraginaceae) Taxa from Turkey
Bangladesh J. Bot. 36(2): 93-103, 2007 (December) MICROMORPHOLOGY AND ANATOMY OF THREE SYMPHYTUM (BORAGINACEAE) TAXA FROM TURKEY OZNUR ERGEN AKCIN AND HILAL BAKI Department of Biology, Sciences & Arts Faculty, Ordu University, Ordu, Turkey E-mail: [email protected] Key words: Micromorphology, Anatomy, Symphytum Abstract Symphytum asperum Lepechin, S. ibericum Steven and S. sylvaticum Boiss. were examined morphologically, micromorphologically and anatomically. Scanning electron microscopy was used to examine leaf surface and trichomes of these species. These species had bifacial and hypostomatous leaf types. Epidermal cells of leaves were usually polygonal or irregular in form. The pattern of anticlinical cells may vary in different species and between the upper and lower epidermis of the same species. Stomata are anisocytic and anomocytic in three species. Stomata index is 27.5 for S. sylvaticum, 24.65 for S. ibericum and 21.86 for S. asperum glandular trichomes are capitate in forms and more dense on the lower epidermis than upper epidermis. Eglandular trichomes are simple, short or long, unicellular or multicellular and thin or thick. Introduction Symphytum L. (Boraginaceae) is represented by about 23 taxa in Turkey (Wickens 1978, Yildirimli 2000). Symphytum species are common garden plants and have been used as a source of herbal medicines for >2000 years. Leaf and root of Symphytum species are used by lay public, herbalists and physicians for treatment of broken bones, tendon damage, ulcerations in the gastrointestinal tract, and lung congestion. These species are also rich in many crucial nutrients, such as protein, antioxidant, vitamins and vitamin B12, and are common component in the diet of certain ethnic groups (Hills 1976, Rode 2002). -
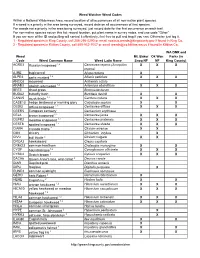
Trail Weed Watcher Weed Check List
Weed Watcher Weed Codes Within a National Wilderness Area, record location of all occurrences of all non-native plant species. If a weed is a priority in the area being surveyed, record data on all occurrences of that species. For weeds not a priority in the area being surveyed, just record data for the first occurrence on each trail. For non-native species not on this list: record location, put plant name in survey notes, and use code "Other" If you are sure of the ID and pulling will control it effectively, feel free to pull and bag if you can. Otherwise just log it. 1 - Regulated species in King County, call 206-296-0290 or email [email protected] if found in King Co. 2 - Regulated species in Kittitas County, call 509-962-7007 or email [email protected] if found in Kittitas Co. WA DNR and Weed Mt. Baker Ok Wen Parks (in Code Weed Common Name Weed Latin Name Snoq NF NF King County) ACRE3 Russian knapweed 1,2 Centaurea repens (Acroptilon XX X repens) AJRE bugleweed Ajuga reptans X ALPE4 garlic mustard 1,2 Alliaria petiolata XX X ANCO2 mayweed Anthemis cotula ARAB3 absinth wormwood 1,2 Artemisia absinthium XX X BRTE cheat grass Bromus tectorum BUDA2 butterfly bush Buddleia davidii X X CANU5 musk thistle 1,2 Carduus nutans XX X CASE13 hedge bindweed or morning glory Calystegia sepium X X CEDI3 diffuse knapweed 1,2 Centaurea diffusa XX X CEER5 European centaury Centaurium erythraea CEJA brown knapweed 1,2 Centaurea jacea XX X CEPR2 meadow knapweed 1,2 Centaurea pratensis XX X CEST8 spotted knapweed 1,2 Centaurea stoebe -

Boraginaceae) with New First Record of the Species Symphytum Tuberosum L
Plant Archives Vol. 18 No. 2, 2018 pp. 2068-2076 e-ISSN:2581-6063 (online), ISSN:0972-5210 A COMPARATIVE SYSTEMATIC STUDY OF THE GENUS SYMPHYTUM L. (BORAGINACEAE) WITH NEW FIRST RECORD OF THE SPECIES SYMPHYTUM TUBEROSUM L. FROM IRAQ. Adel Mohan Aday Al-Zubaidy* and Sherzad Rasul Abdalla Tobakari Plant Production Department, Technical College of Applied Sciences-Sulaimani Polytechnic University-Iraq Abstract The current study includes a comparative morphological study of the genus Symphytum L. within the family Boraginaceae in relation to the phenotypic study, the study of the external manifestations of pollen, the environment and geographical distribution. The study included the study of the characteristics of roots, stems, leaves, flowers, inflorescence, fruits and nutlets. Variation in characteristics was discussed and it was noted that the characteristics of flowers were more important in taxonomic terms in identification and isolating studied species. The study also indicated that the pollen of all studied species varies in form and size and have characteristics of taxonomic significance that may be adopted in the isolation and diagnosis of these species which studied for the first time in Iraq. The research has succeeded in surveying the Iraqi geographical districts to reveal the distribution of these taxa. Therefore, the researcher obtained a large number of samples and new sites were identified for the distribution of the studied taxa. All samples collected and those recorded in the Iraqi herbaria were studied. Symphytum kurdicum Boiss. & Hausskn in Boiss. was found to be widely distribution, while Symphytum tuberosum L. was limited. Based on morphological and pollen characteristics of the studied taxa, Symphytum tuberosum was first recorded in refined in this study as it was added as a new first record of this species to flora of Iraq. -

Alaska Non-Native Plant Invasiveness Ranking Form
ALASKA NON-NATIVE PLANT INVASIVENESS RANKING FORM Botanical name: Symphytum officinale L. Common name: common comfrey Assessors: Timm Nawrocki Lindsey A. Flagstad Research Technician Research Technician Alaska Natural Heritage Program, University of Alaska Alaska Natural Heritage Program, University of Alaska Anchorage, Anchorage, 707 A Street, 707 A Street, Anchorage, Alaska 99501 Anchorage, Alaska 99501 (907) 257-2798 (907) 257-2786 Matthew L. Carlson, Ph.D. Associate Professor Alaska Natural Heritage Program, University of Alaska Anchorage, 707 A Street, Anchorage, Alaska 99501 (907) 257-2790 Reviewers: Ashley Grant Bonnie M. Million. Invasive Plant Program Instructor Alaska Exotic Plant Management Team Liaison Cooperative Extension Service, University of Alaska Alaska Regional Office, National Park Service, U.S. Fairbanks Department of the Interior 1675 C Street, 240 West 5th Avenue Anchorage, Alaska 99501 Anchorage, Alaska 99501 (907) 786-6315 (907) 644-3452 Gino Graziano Natural Resource Specialist Plant Materials Center, Division of Agriculture, Department of Natural Resources, State of Alaska 5310 S. Bodenburg Spur, Palmer, Alaska 99645 (907) 745-4469 Date: 10/8/2010 Date of previous ranking, if any: 4T OUTCOME SCORE: CLIMATIC COMPARISON This species is present or may potentially establish in the following eco-geographic regions: Pacific Maritime Yes Interior-Boreal Yes Arctic-Alpine Yes INVASIVENESS RANKING Total (total answered points possible1) Total Ecological impact 40 (40) 16 Biological characteristics and dispersal ability 25 (25) 12 Ecological amplitude and distribution 25 (25) 13 Feasibility of control 10 (10) 7 Outcome score 100 (100)b 48a Relative maximum score2 48 1 For questions answered “unknown” do not include point value for the question in parentheses for “total answered points possible.” 2 Calculated as a/b × 100 A. -

Using Frontier Technologies for the Quality Assurance of Medicinal Herbs
Using Frontier Technologies for the Quality Assurance of Medicinal Herbs RIRDC Publication No. 11/093 RIRDCInnovation for rural Australia Using Frontier Technologies for the Quality Assurance of Medicinal Herbs by Associate-Professor Eddie Pang November 2011 RIRDC Publication No. 11/093 RIRDC Project No. PRJ-000763 © 2011 Rural Industries Research and Development Corporation. All rights reserved. ISBN 978-1-74254-273-7 ISSN 1440-6845 Using Frontier Technologies for the Quality Assurance of Medicinal Herbs Publication No. 11/093 Project No. PRJ-000763 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. This publication is copyright. -

Ethnoveterinary Medicines Used for Ruminants in British Columbia, Canada Cheryl Lans1, Nancy Turner*2, Tonya Khan3, Gerhard Brauer4 and Willi Boepple5
Journal of Ethnobiology and Ethnomedicine BioMed Central Research Open Access Ethnoveterinary medicines used for ruminants in British Columbia, Canada Cheryl Lans1, Nancy Turner*2, Tonya Khan3, Gerhard Brauer4 and Willi Boepple5 Address: 1BCICS, University of Victoria, British Columbia, V8W 2Y2, Canada, 2School of Environmental Studies, University of Victoria, British Columbia, V8W 3P5, Canada, 3DVM, Vancouver, British Columbia, Canada, 4School of Health Information Science, University of Victoria, British Columbia, V8W 3P5, Canada and 5Canadian Liaison National Saanen Breeders. 499 Millstream Lake Rd. Victoria, B.C., Canada, V9E 1K2 Email: Cheryl Lans - [email protected]; Nancy Turner* - [email protected]; Tonya Khan - [email protected]; Gerhard Brauer - [email protected]; Willi Boepple - [email protected] * Corresponding author Published: 26 February 2007 Received: 19 December 2006 Accepted: 26 February 2007 Journal of Ethnobiology and Ethnomedicine 2007, 3:11 doi:10.1186/1746-4269-3-11 This article is available from: http://www.ethnobiomed.com/content/3/1/11 © 2007 Lans et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: The use of medicinal plants is an option for livestock farmers who are not allowed to use allopathic drugs under certified organic programs or cannot afford to use allopathic drugs for minor health problems of livestock. Methods: In 2003 we conducted semi-structured interviews with 60 participants obtained using a purposive sample. Medicinal plants are used to treat a range of conditions. -

Buffer Handbook Plant List
THE BUFFER HANDBOOK PLANT LIST Originally Developed by: Cynthia Kuhns, Lake & Watershed Resource Management Associates With funding provided by U.S. Environmental Protection Agency and Maine Department of Environmental Protection,1998. Revised 2001 and 2009. Publication #DEPLW0094-B2009 TABLE OF CONTENTS Page Acknowledgements 1 Introductory Information Selection of Plants for This List 1 Plant List Organization & Information 3 Terms & Abbreviations 4 Plant Hardiness Zone Map 5 General Tree & Shrub Planting Guidelines 5 Tips for Planting Perennials 7 Invasive Plants to Avoid 7 Plant Lists TREES 8 (30 to 100 ft.) SHRUBS 14 Small Trees/Large Shrubs 15 (12 to 30 ft.) Medium Shrubs 19 (6 to 12 ft.) Small Shrubs 24 (Less than 6 ft.) GROUNDLAYERS 29 Perennial Herbs & Flowers 30 Ferns 45 Grasses 45 Vines 45 References 49 ACKNOWLEDGEMENTS Original Publication: This plant list was published with the help of Clean Water Act, Section 319 funds, under a grant awarded to the Androscoggin Valley Soil and Water Conservation District and with help from the Maine Department of Environmental Protection and the U.S. Environmental Protection Agency. Graphics and ‘clip-art’ used in this document came from the University of Wisconsin-Extension and from Microsoft Office 97(Small Business Edition) and ClickArt 97 (Broderbund Software, Inc). This publication was originally developed by Cynthia Kuhns of Lake & Watershed Resource Management Associates. Substantial assistance was received from Phoebe Hardesty of the Androscoggin Valley Soil and Water Conservation District. Valuable review and advice was given by Karen Hahnel and Kathy Hoppe of the Maine Department of Environmental Protection. Elizabeth T. Muir provided free and cheerful editing and botanical advice.