Sterol Addition During Pollen Collection by Bees
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional Morphology and Evolution of the Sting Sheaths in Aculeata (Hymenoptera) 325-338 77 (2): 325– 338 2019
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2019 Band/Volume: 77 Autor(en)/Author(s): Kumpanenko Alexander, Gladun Dmytro, Vilhelmsen Lars Artikel/Article: Functional morphology and evolution of the sting sheaths in Aculeata (Hymenoptera) 325-338 77 (2): 325– 338 2019 © Senckenberg Gesellschaft für Naturforschung, 2019. Functional morphology and evolution of the sting sheaths in Aculeata (Hymenoptera) , 1 1 2 Alexander Kumpanenko* , Dmytro Gladun & Lars Vilhelmsen 1 Institute for Evolutionary Ecology NAS Ukraine, 03143, Kyiv, 37 Lebedeva str., Ukraine; Alexander Kumpanenko* [[email protected]]; Dmytro Gladun [[email protected]] — 2 Natural History Museum of Denmark, SCIENCE, University of Copenhagen, Universitet- sparken 15, DK-2100, Denmark; Lars Vilhelmsen [[email protected]] — * Corresponding author Accepted on June 28, 2019. Published online at www.senckenberg.de/arthropod-systematics on September 17, 2019. Published in print on September 27, 2019. Editors in charge: Christian Schmidt & Klaus-Dieter Klass. Abstract. The sting of the Aculeata or stinging wasps is a modifed ovipositor; its function (killing or paralyzing prey, defense against predators) and the associated anatomical changes are apomorphic for Aculeata. The change in the purpose of the ovipositor/sting from being primarily an egg laying device to being primarily a weapon has resulted in modifcation of its handling that is supported by specifc morphological adaptations. Here, we focus on the sheaths of the sting (3rd valvulae = gonoplacs) in Aculeata, which do not penetrate and envenom the prey but are responsible for cleaning the ovipositor proper and protecting it from damage, identifcation of the substrate for stinging, and, in some taxa, contain glands that produce alarm pheromones. -
Weed Check List Survey Date
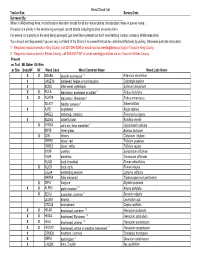
Weed Check List Trail or Site: Survey Date: Surveyed By: When in Wildnerness Area, record location and other details for all non-native plants. Include plant name in survey notes. If a weed is a priority in the area being surveyed, record details including location on survey form. For weeds not a priority in the area being surveyed, just mark them present but don't need lat/long location unless in Wilderness Area. You can pull and bag weeds if you are very confident of the ID and it is a weed that can be controlled effectively by pulling. Otherwise just note its location. 1 - Regulated noxious weeds in King County, call 206-296-0290 or email [email protected] if found in King County 2 - Regulated noxious weed in Kittitas County, call 509-962-7007 or email [email protected] if found in Kittitas County Present on Trail Mt. Baker Ok Wen or Site Snoq NF NF Weed Code Weed Common Name Weed Latin Name XXARAB3 absinth wormwood 1,2 Artemisia absinthium X CASE13 bindweed, hedge or morning glory Calystegia sepium X SODU bittersweet nightshade Solanum dulcamara XXRULA blackberry, evergreen or cutleaf 2 Rubus laciniatus XXRUAR9 blackberry, Himalayan 2 Rubus armeniacus SILA21 bladder campion 2 Silene latifolia X AJRE bugleweed Ajuga reptans RARE3 buttercup, creeping Ranunculus repens X BUDA2 butterfly bush Buddleia davidii X HYRA3 cat’s ear, false dandelion 2 Hypochaeris radicata BRTE cheat grass Bromus tectorum X CIIN chicory Cichorium intybus TRPR2 clover, red Trifolium pratense TRRE3 clover, white Trifolium repens SYOF comfrey Symphytum officinale TAOF dandelion Taraxacum officinale RUOB dock, broadleaf Rumex obtusifolius X RUCR dock, curly Rumex crispus LALA4 everlasting peavine Lathyrus latifolius MAPE2 false mayweed Tripleurospermum perforatum X DIPU foxglove Digitalis purpurea XXALPE4 garlic mustard 1,2 Alliaria petiolata X SEVU groundsel, common 2 Senecio vulgaris LEONT hawkbit Leontodon spp. -

Suitability of Root and Rhizome Anatomy for Taxonomic
Scientia Pharmaceutica Article Suitability of Root and Rhizome Anatomy for Taxonomic Classification and Reconstruction of Phylogenetic Relationships in the Tribes Cardueae and Cichorieae (Asteraceae) Elisabeth Ginko 1,*, Christoph Dobeš 1,2,* and Johannes Saukel 1,* 1 Department of Pharmacognosy, Pharmacobotany, University of Vienna, Althanstrasse 14, Vienna A-1090, Austria 2 Department of Forest Genetics, Research Centre for Forests, Seckendorff-Gudent-Weg 8, Vienna A-1131, Austria * Correspondence: [email protected] (E.G.); [email protected] (C.D.); [email protected] (J.S.); Tel.: +43-1-878-38-1265 (C.D.); +43-1-4277-55273 (J.S.) Academic Editor: Reinhard Länger Received: 18 August 2015; Accepted: 27 May 2016; Published: 27 May 2016 Abstract: The value of root and rhizome anatomy for the taxonomic characterisation of 59 species classified into 34 genera and 12 subtribes from the Asteraceae tribes Cardueae and Cichorieae was assessed. In addition, the evolutionary history of anatomical characters was reconstructed using a nuclear ribosomal DNA sequence-based phylogeny of the Cichorieae. Taxa were selected with a focus on pharmaceutically relevant species. A binary decision tree was constructed and discriminant function analyses were performed to extract taxonomically relevant anatomical characters and to infer the separability of infratribal taxa, respectively. The binary decision tree distinguished 33 species and two subspecies, but only five of the genera (sampled for at least two species) by a unique combination of hierarchically arranged characters. Accessions were discriminated—except for one sample worthy of discussion—according to their subtribal affiliation in the discriminant function analyses (DFA). However, constantly expressed subtribe-specific characters were almost missing and even in combination, did not discriminate the subtribes. -

Towards an Updated Checklist of the Libyan Flora
Towards an updated checklist of the Libyan flora Article Published Version Creative Commons: Attribution 3.0 (CC-BY) Open access Gawhari, A. M. H., Jury, S. L. and Culham, A. (2018) Towards an updated checklist of the Libyan flora. Phytotaxa, 338 (1). pp. 1-16. ISSN 1179-3155 doi: https://doi.org/10.11646/phytotaxa.338.1.1 Available at http://centaur.reading.ac.uk/76559/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.11646/phytotaxa.338.1.1 Identification Number/DOI: https://doi.org/10.11646/phytotaxa.338.1.1 <https://doi.org/10.11646/phytotaxa.338.1.1> Publisher: Magnolia Press All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Phytotaxa 338 (1): 001–016 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.338.1.1 Towards an updated checklist of the Libyan flora AHMED M. H. GAWHARI1, 2, STEPHEN L. JURY 2 & ALASTAIR CULHAM 2 1 Botany Department, Cyrenaica Herbarium, Faculty of Sciences, University of Benghazi, Benghazi, Libya E-mail: [email protected] 2 University of Reading Herbarium, The Harborne Building, School of Biological Sciences, University of Reading, Whiteknights, Read- ing, RG6 6AS, U.K. -

Milk Thistle
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control BIOLOGY AND BIOLOGICAL CONTROL OF EXOTIC T RU E T HISTL E S RACHEL WINSTON , RICH HANSEN , MA R K SCH W A R ZLÄNDE R , ER IC COO M BS , CA R OL BELL RANDALL , AND RODNEY LY M FHTET-2007-05 U.S. Department Forest September 2008 of Agriculture Service FHTET he Forest Health Technology Enterprise Team (FHTET) was created in 1995 Tby the Deputy Chief for State and Private Forestry, USDA, Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ On the cover: Italian thistle. Photo: ©Saint Mary’s College of California. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410 or call 202-720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. The use of trade, firm, or corporation names in this publication is for information only and does not constitute an endorsement by the U.S. -

A Baseline Invertebrate Survey of the Ken Hill Estate, 2019
A baseline invertebrate survey of the Ken Hill Estate, 2019 Graeme Lyons February 2020 Fig. 1. The nationally rare Breckland Leather Arenocoris waltlii is listed as Critically Endangered 0 – Summary The Ken Hill Estate plan to rewild a large area of some 400 ha of their Estate during 2019 and 2020. The summer of 2019 was the last crop for much of this area and as such, the 2019 survey season was an exciting opportunity to collect baseline data before any changes were made to the site. The author was commissioned to carry out a wide range of surveys in 2019, including this baseline invertebrate survey. A methodology used by the author to monitor other rewilding sites nationally was adopted based upon surveying eight fields/sections, six times from April to September. The sections needed to represent the site geographically, representationally in terms of habitats and crops and make a realistic circular route. Each section was recorded for 30 minutes using the method pertinent to the season. Specimens were taken and identified at the microscope. Eight species lists were produced and an overall site species list was also produced. All species with conservation status were recorded and species accounts given. Any species recorded between section or on different surveys were also recorded. A total of 1895 records were made during the six visits comprised of 811 species, 50 species of which had conservation status (6.2%). The total number of species was exceptionally rich, the highest figure of any six-visit invertebrate survey carried out by the author. The proportion of species with status was comparable to other rewilding surveys but these were carried out some 15 years after rewilding began. -

Dasypoda Morawitzi
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Ampulex - Zeitschrift für aculeate Hymenopteren Jahr/Year: 2017 Band/Volume: 9 Autor(en)/Author(s): Schmid-Egger Christian, Dubitzky Andreas Artikel/Article: Dasypoda morawitzi (Radschenko, 2016) neu für die Fauna von Mitteleuropa (Hymenoptera, Apoidea) 27-31 ©Ampulex online, Christian Schmid-Egger; download unter http://www.ampulex.de oder www.zobodat.at Schmid-Egger & Dubitzky: Dasypoda morawitzi neu für Mitteleuropa AMPULEX 9|2017 Dasypoda morawitzi (Radschenko, 2016) neu für die Fauna von Mitteleuropa (Hymenoptera, Apoidea) Christian Schmid-Egger1, Andreas Dubitzky2 1 Fischerstraße 1 | 10317 Berlin | Germany | [email protected] | www.bembix.de 2 Am Höllberg 13 | 85241 Hebertshausen | Germany | [email protected] Zusammenfassung Im vorliegenden Artikel werden neue und aktuelle Funde der kürzlich aus der Ukrainie, Russland und angrenzender Länder neu be- schriebenen Dasypoda morawitzi vorgestellt. Die Art wird erstmals aus Deutschland, Österreich und Bulgarien nachgewiesen. Das bisher bekannte Verbreitungsgebiet erweitert sich damit um rund 1100 Kilometer nach Westen. Zudem werden Unterscheidungs- merkmale zur nahe verwandten Art Dasypoda hirtipes genannt, mit der sie bisher vermengt wurde. Die aus Mitteleuropa bekannten Biotope werden beschrieben, die vermutete Oligolektie auf Korbblütler wird bestätigt. Die Art ist bisher sowohl in Deutschland als auch in Österreich nur von jeweils einem Standort im extremen Osten beider Länder nachgewiesen. Summary Christian Schmid-Egger & Andreas Dubitzky: Dasypoda morawitzi (Radschenko, 2016) first record in Central Europe (Hymenopte- ra, Apoidea). Recently, Dasypoda morawitzi was described from Ukraine, Russia and adjacent countries. The species is now also recor- ded from Germany, Austria and Bulgaria. Diagnostic recognition characters are provided. -

An Empirical Assessment of a Single Family‐Wide Hybrid Capture Locus
APPLICATION ARTICLE An empirical assessment of a single family-wide hybrid capture locus set at multiple evolutionary timescales in Asteraceae Katy E. Jones1,14 , Tomáš Fér2 , Roswitha E. Schmickl2,3 , Rebecca B. Dikow4 , Vicki A. Funk5 , Sonia Herrando-Moraira6 , Paul R. Johnston7,8,9, Norbert Kilian1 , Carolina M. Siniscalchi10,11 , Alfonso Susanna6 , Marek Slovák2,12 , Ramhari Thapa10,11, Linda E. Watson13 , and Jennifer R. Mandel10,11 Manuscript received 27 February 2019; revision accepted PREMISE: Hybrid capture with high-throughput sequencing (Hyb-Seq) is a powerful tool for 5 September 2019. evolutionary studies. The applicability of an Asteraceae family-specific Hyb-Seq probe set and 1 Botanischer Garten und Botanisches Museum Berlin, Freie the outcomes of different phylogenetic analyses are investigated here. Universität Berlin, Königin-Luise-Str. 6–8, 14195 Berlin, Germany 2 Department of Botany, Faculty of Science, Charles University, METHODS: Hyb-Seq data from 112 Asteraceae samples were organized into groups at differ- Benátská 2, CZ 12800 Prague, Czech Republic ent taxonomic levels (tribe, genus, and species). For each group, data sets of non-paralogous 3 Institute of Botany, The Czech Academy of Sciences, Zámek 1, CZ loci were built and proportions of parsimony informative characters estimated. The impacts 25243 Průhonice, Czech Republic of analyzing alternative data sets, removing long branches, and type of analysis on tree reso- 4 Data Science Lab, Office of the Chief Information lution and inferred topologies were investigated in tribe Cichorieae. Officer, Smithsonian Institution, Washington, D.C. 20013-7012, USA RESULTS: Alignments of the Asteraceae family-wide Hyb-Seq locus set were parsimony infor- 5 Department of Botany, National Museum of Natural mative at all taxonomic levels. -

“Study of the Effects of an Artificial Cut on the Invasive Plant S. Ineaquidens”
UNIVERSITAT POLITÈCNICA DE VALÈNCIA E S C O L A POLITÈCNICA SUPERIOR D E G A N D I A Grau en Ciències Ambientals “Study of the effects of an artificial cut on the invasive plant S. Ineaquidens” TREBALL FINAL DE GRAU Autor/a: Marc Fuster Adrover Tutor/s: Prof. Dr. Martin Diekmann Prof. Dra. Maria Pilar Donat Torres GANDIA, 2016 2 3 I ABSTRACT .......................................................................................................... 5 1 INTRODUCTION ................................................................................................. 6 2 MATERIAL AND METHODS ............................................................................. 9 2.1 STUDY SITE AND PLANT SAMPLING ........................................................................ 9 2.2 PLANT TREATMENT AND ANALYSIS ...................................................................... 10 2.3 STATISTICAL ANALYSIS ........................................................................................ 11 3 RESULTS .......................................................................................................... 12 3.1 CONSEQUENCES OF CUT ON INDIVIDUAL SURVIVAL OF S. INEAQUIDENS ........... 12 3.2 CONSEQUENCES OF CUT ON GROWTH OF S. INEAQUIDENS ................................ 13 3.3 CONSEQUENCES OF CUT ON FLOWER PRODUCTION OF S. INEAQUIDENS .......... 17 4 DISCUSSION .................................................................................................... 19 4.1 METHODOLOGY ................................................................................................... -

Coastal Invasive Plant Management Strategy ______
Coastal Invasive Plant Management Strategy Prepared by Brian Wikeem, P.Ag. and Sandra Wikeem Solterra Resources Inc . June 30, 2010 ACKNOWLEDGEMENTS The BC Agricultural Research and Development Corporation and the BC Ministry of Transportation and Infrastructure are gratefully acknowledged for financial support for this project. In-kind support was also provided by the BC Ministry of Agriculture and Lands, BC Ministry of Environment, and the BC Ministry of Forests and Range. The members of the Coastal Invasive Plant Committee board of directors including Becky Brown, Glenda Barr, Zak Henderson, Michele Jones, Rob Lawrence, Kate Miller, June Pretzer, Valentin Schaefer, and Ernie Sellentin are thanked for their contributions to this report. Lynn Atwood, past Program Coordinator, is thanked for providing unpublished reports that furnished background information. Jeff Hallworth and Melissa Noel are especially acknowledged for collecting material, reviewing drafts of the report, and overall support. Coastal Invasive Plant Management Strategy ___________________________________________________________________________ EXECUTIVE SUMMARY Invasive plants have been a problem in Coastal British Columbia (BC) since earliest European settlement but little has been done to control these species until recently. The Coastal Invasive Plant Committee (CIPC) was formed in 2005 to service Vancouver Island and surrounding coastal communities. The committee consists of public and private sector groups, First Nations, industry, utilities, and conservation groups that share a common interest in promoting coordination and cooperation to manage invasive plants in the region. The CIPC area covers approximately 60,000 km 2 including Vancouver Island, mainland coast and Gulf Islands; and consists of eight regional districts, 34 municipalities, 15 Gulf Islands, and 57 First Nations. -

First Steps Towards a Floral Structural Characterization of the Major Rosid Subclades
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2006 First steps towards a floral structural characterization of the major rosid subclades Endress, P K ; Matthews, M L Abstract: A survey of our own comparative studies on several larger clades of rosids and over 1400 original publications on rosid flowers shows that floral structural features support to various degrees the supraordinal relationships in rosids proposed by molecular phylogenetic studies. However, as many apparent relationships are not yet well resolved, the structural support also remains tentative. Some of the features that turned out to be of interest in the present study had not previously been considered in earlier supraordinal studies. The strongest floral structural support is for malvids (Brassicales, Malvales, Sapindales), which reflects the strong support of phylogenetic analyses. Somewhat less structurally supported are the COM (Celastrales, Oxalidales, Malpighiales) and the nitrogen-fixing (Cucurbitales, Fagales, Fabales, Rosales) clades of fabids, which are both also only weakly supported in phylogenetic analyses. The sister pairs, Cucurbitales plus Fagales, and Malvales plus Sapindales, are structurally only weakly supported, and for the entire fabids there is no clear support by the present floral structural data. However, an additional grouping, the COM clade plus malvids, shares some interesting features but does not appear as a clade in phylogenetic analyses. Thus it appears that the deepest split within eurosids- that between fabids and malvids - in molecular phylogenetic analyses (however weakly supported) is not matched by the present structural data. Features of ovules including thickness of integuments, thickness of nucellus, and degree of ovular curvature, appear to be especially interesting for higher level relationships and should be further explored. -

REVIEWS Algeria, Egypt, France, Greece, Iran, Iraq, I Srael, Itaiy, Jordan, Lebanon, Libya, Morocco, Spain, Syria, Turkey and Yugos Lavia
178 Plant ProtectIOn Quanerly Vol . 2(4) 1987 Distribution R. lutea is indigenous [0 the Medit erranean Basin and Asia Minor, occurring in REVIEWS Algeria, Egypt, France, Greece, Iran, Iraq, i srael, itaiy, Jordan, Lebanon, Libya, Morocco, Spain, Syria, Turkey and Yugos lavia. It has spread widely around the world and has been recorded in Australia, Austria, Belgium, Czechoslovakia, Den mark, Finland, Germany, Great Britain, Hungary, Norway, Romania, Sweden, Switzerland, U.S.A. and U.S.S.R. (Abdal· iah and De Wi. 1978). Although R. Iwea is present in Queens The Biology of Australian Weeds land, New South Wales, Victoria and Tas 17. Reseda lutea L. mania (Figure 2) , it has not become an important weed there. There is only a single record rrom Western Australi a (Dalwal linu) (Pearce 1982). R. lutea is round only rarely on the Tas manian main land. but is more common on Fiinders Island (8. Hyde-Wyau, pers. comm.). In South Australia, R. lutea is a serious pest plant wh ich occurs, as eit her sporadic outbreaks or areas or dense inrestations, over most or the agricultural areas such as the lower North, Yorke Peninsula and parts or the Eyre Peninsula. It is a weed whi ch is spreadi ng to new parts within South Australia (Figure 3). J . W. Heap, M. C. Willcocks· and P.M. Kloot Annual rainrall does not appear 10 con SA Department 01 Agricutture. G.P.O. Box 1671 . Adelaide. SA 5001 trol its distribution in the agri cultural areas, • Present address: School 01 Wool and Pastoral Sciences.