14.5 Conversion of Alkynes Into Aldehydes and Ketones 655
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigation of Base-Free Copper-Catalysed Azide–Alkyne Click Cycloadditions (Cuaac) in Natural Deep Eutectic Solvents As Green and Catalytic Reaction Media
Investigation of Base-free Copper-Catalysed Azide–Alkyne Click Cycloadditions (CuAAc) in Natural Deep Eutectic Solvents as Green and Catalytic Reaction Media Salvatore V. Giofrè,1* Matteo Tiecco,2* Angelo Ferlazzo,3 Roberto Romeo,1 Gianluca Ciancaleoni,4 Raimondo Germani2 and Daniela Iannazzo3 1. Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università di Messina, Viale Annunziata, I-98168 Messina, Italy. 2. Dipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, via Elce di Sotto 8, I- 06123 Perugia, Italy. 3. Dipartimento di Ingegneria, Università of Messina, Contrada Di Dio, I-98166 Messina, Italy 4. Dipartimento di Chimica e Chimica Industriale (DCCI), Università di Pisa, Via Giuseppe Moruzzi, 13, I-56124 Pisa, Italy. * Corresponding authors Email addresses: [email protected] (Salvatore V. Giofrè); [email protected] (Matteo Tiecco). ABSTRACT The click cycloaddition reaction of azides and alkynes affording 1,2,3-triazoles is a transformation widely used to obtain relevant products in chemical biology, medicinal chemistry, materials science and other fields. In this work, a set of Natural Deep Eutectic Solvents (NADESs) as “active” reaction media has been investigated in the copper-catalysed azide–alkyne cycloaddition reactions (CuAAc). The use of these green liquids as green and catalytic solvents has shown to improve the reaction effectiveness, giving excellent yields. The NADESs proved to be “active” in this transformation for the absence of added bases in all the performed reactions and in several cases for their reducing capabilities. The results were rationalized by DFT calculations which demonstrated the involvement of H-bonds between DESs and alkynes as well as a stabilization of copper catalytic intermediates. -
Direct Β‑Functionalization of Cyclic Ketones with Aryl Ketones Via the Merger of Photoredox and Organocatalysis Filip R
Communication pubs.acs.org/JACS Direct β‑Functionalization of Cyclic Ketones with Aryl Ketones via the Merger of Photoredox and Organocatalysis Filip R. Petronijevic,́† Manuel Nappi,† and David W. C. MacMillan* Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States *S Supporting Information ABSTRACT: The direct β-coupling of cyclic ketones with aryl ketones has been achieved via the synergistic combination of photoredox catalysis and organocatalysis. Diaryl oxymethyl or aryl−alkyl oxymethyl radicals, transiently generated via single-electron reduction of ketone precursors, readily merge with β-enaminyl radical species, generated by photon-induced enamine oxidation, to produce γ-hydroxyketone adducts. Experimental evidence indicates that two discrete reaction pathways can be operable in this process depending upon the nature of the ketyl radical precursor and the photocatalyst. he direct β-functionalization of saturated ketones and T aldehydes is an important yet elusive goal in organic chemistry.1 While carbonyl groups are readily amenable to ipso- and α-carbon substitution with a range of nucleophiles and electrophiles respectively,2,3 activation at the β-methylene position poses a significant synthetic challenge. With a few notable exceptions,1,4 carbonyl β-functionalization has tradition- ally been restricted to the conjugate addition of soft nucleophiles are accessed via carbene catalysis,9,10 nucleophilic addition of into α,β-unsaturated carbonyl systems. As such, the development acetal-protected Grignard reagents,11 or stoichiometric metal- 5 − of a general catalytic platform for the direct β-functionalization activated homoenolate equivalents.12 16 of saturated ketones or aldehydes would represent a conceptual Drawing from the mechanistic insights gained in the course of and practical advance for the field. -
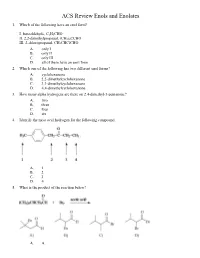
Ochem ACS Review 18 Enols and Enolates
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

Aldehydes and Ketones Are Simple Organic Compounds Containing a Carbonyl Group
Aldehydes and Ketones are simple organic compounds containing a carbonyl group. Carbonyl group contains carbon- oxygen double bond. These organic compounds are simple because the carbon atom presents in the carbonyl group lack reactive groups such as OH or Cl. By Dr. Sayed Hasan Mehdi Assistant Professor Department of Chemistry Shia P.G. College, Lucknow Dr. S.Hasan Mehdi 6/13/2020 This is to bring to kind notice that the matter of this e- content is for the B.Sc. IV semester students. It has been taken from the following sources. The students are advised to follow these books as well. •A TEXTBOOK OF ORGANIC CHEMISTRY by Arun Bahl & B.S. Bahl, S. Chand & Company Ltd. Publication. •Graduate Organic Chemistry by M. K. Jain and S.C. Sharma, Vishal Publishing Co. •Pradeep’s Organic Chemistry Vol II by R. N. Dhawan, Pradeep Publication, Jalandhar. Dr. S.Hasan Mehdi 6/13/2020 An aldehyde is one of the classes of carbonyl group- containing alkyl group on one end and hydrogen on the other end. The R and Ar denote alkyl or aryl member respectively. In the condensed form, the aldehyde is written as –CHO. Dr. S.Hasan Mehdi 6/13/2020 Dr. S.Hasan Mehdi 6/13/2020 1. From Alcohols: a. By oxidation of Alcohols: Aldehydes and ketones can be prepared by the controlled oxidation of primary and secondary alcohols using an acidified solution of potassium dichromate or permanganate. Primary alcohol produces aldehydesRef. Last slide. O K Cr O RCH2OH + [O] 2 2 7 + R C H H 10 Alcohol Aldehyde O CH3CH2OH+ [O] K2Cr2O7 + CH3 C H H Ethyl Alcohol Acetaldehyde The aldehydes formed in the above reaction are very easily oxidised to carboxylc acids if allowed to remain in the reaction mixture. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Complexes for Click Azide-Alkyne Cycloaddition Reactions
Well-defined Copper(I) Complexes for Click Azide-Alkyne Cycloaddition Reactions: One Click Beyond Silvia Díez-González* Department of Chemistry, Imperial College London Exhibition Road, South Kensington, SW7 2AZ London (UK) [email protected] Abstract: The discovery of copper(I) species as excellent catalysts for the regioselective cycloaddition reactions of azides and alkynes served as proof-of-concept of the importance of Click chemistry and opened a broad field of research that has found numerous ramifications in biochemistry, materials and medicinal science, for instance. The use of ligands in this context not only serves to stabilize the oxidation state of copper, but has also been shown to increase and modulate its reactivity. Still, efforts focused on developing more efficient catalytic systems for this transformation remain limited. Herein, the catalytic activities of ligated copper systems are reviewed in a way intended inspirational for further developments. 1. Introduction Transition metal catalysis is one of the most powerful tools available to chemists for the development of cleaner and more sustainable processes. Although simple metal salts can mediate a number of transformations, it was the use of ligands that catapulted organometallic catalysis to its present leading status. In particular, the use of well-defined complexes allows for a better control of the nature of the species present in the reaction media and generally avoids the need for excess of ligand and/or reagents for achieving optimal catalytic performance. These two points are crucial in the continuous quest of molecular chemistry for more efficient and less contaminating processes capable of providing diverse and complex architectures. -

Chapter 14 – Aldehydes and Ketones
Chapter 14 – Aldehydes and Ketones 14.1 Structures and Physical Properties of Aldehydes and Ketones Ketones and aldehydes are related in that they each possess a C=O (carbonyl) group. They differ in that the carbonyl carbon in ketones is bound to two carbon atoms (RCOR’), while that in aldehydes is bound to at least one hydrogen (H2CO and RCHO). Thus aldehydes always place the carbonyl group on a terminal (end) carbon, while the carbonyl group in ketones is always internal. Some common examples include (common name in parentheses): O O H HH methanal (formaldehyde) trans-3-phenyl-2-propenal (cinnamaldehyde) preservative oil of cinnamon O O propanone (acetone) 3-methylcyclopentadecanone (muscone) nail polish remover a component of one type of musk oil Simple aldehydes (e.g. formaldehyde) typically have an unpleasant, irritating odor. Aldehydes adjacent to a string of double bonds (e.g. 3-phenyl-2-propenal) frequently have pleasant odors. Other examples include the primary flavoring agents in oil of bitter almond (Ph- CHO) and vanilla (C6H3(OH)(OCH3)(CHO)). As your book says, simple ketones have distinctive odors (similar to acetone) that are typically not unpleasant in low doses. Like aldehydes, placing a collection of double bonds adjacent to a ketone carbonyl generally makes the substance more fragrant. The primary flavoring agent in oil of caraway is just a such a ketone. 2 O oil of carraway Because the C=O group is polar, small aldehydes and ketones enjoy significant water solubility. They are also quite soluble in typical organic solvents. 14.2 Naming Aldehydes and Ketones Aldehydes The IUPAC names for aldehydes are obtained by using rules similar to those we’ve seen for other functional groups (e.g. -

Chapter 8 - Alkynes: an Introduction to Organic Synthesis
Chapter 8 - Alkynes: An Introduction to Organic Synthesis Draw structures corresponding to each of the following names. 1. ethynylcyclopropane Answer: CCH 2. 3,10-dimethyl-6-sec-butylcyclodecyne Answer: 3. 4-bromo-3,3-dimethyl-1-hexen-5-yne CH3 Br Answer: H 2C CH C CH C C H CH3 4. acetylene Answer: H CCH Provide names for each compound below. CH3 5. CH3C CCHCH2CH2CH3 Answer: 4-methyl-2-heptyne CH 3 6. CCH Answer: 1-ethynyl-2-methylcyclopentane Test Items for McMurry’s Organic Chemistry, Seventh Edition 59 The compound below has been isolated from the safflower plant. Consider its structure to answer the following questions. H H CCCCCCCC H H3C C C C H H C H H 7. What is the molecular formula for this natural product? Answer: C13H10 8. What is the degree of unsaturation for this compound? Answer: We can arrive at the degree of unsaturation for a structure in two ways. Since we know that the degree of unsaturation is the number of rings and/or multiple bonds in a compound, we can simply count them. There are three double bonds (3 degrees) and three triple bonds (six degrees), so the degree of unsaturation is 9. We can verify this by using the molecular formula, C13H10, to calculate a degree of unsaturation. The saturated 13-carbon compound should have the base formula C13H28, so (28 - 10) ÷ 2 = 18 ÷ 2 = 9. 9. Assign E or Z configuration to each of the double bonds in the compound. Answer: H H E CCCCCCCCE H H3C C C C H H C H H 10. -
 in THF](https://docslib.b-cdn.net/cover/3589/hydrogenationn-of-4-octyne-catalyzedd-by-pd-m-w-cf3-2c6h3-bian-ma-in-thf-533589.webp)
Hydrogenationn of 4-Octyne Catalyzedd by Pd[(M^W'- (CF3)2C6H3)) Bian](Ma) in THF
UvA-DARE (Digital Academic Repository) Palladium-catalyzed stereoselective hydrogenation of alkynes to (Z)-alkenes in common solvents and supercritical CO2 Kluwer, A.M. Publication date 2004 Link to publication Citation for published version (APA): Kluwer, A. M. (2004). Palladium-catalyzed stereoselective hydrogenation of alkynes to (Z)- alkenes in common solvents and supercritical CO2. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:04 Oct 2021 5 Kineti5 cc study and Spectroscopic Investigationn of the semi- hydrogenationn of 4-octyne catalyzedd by Pd[(m^w'- (CF3)2C6H3)) bian](ma) in THF 5.11 Introduction Homogeneouss hydrogenation by transition metal complexes has played a key role in the fundamental understandingg of catalytic reactions and has proven to be of great utility in practical applications. -

Strategies for the Synthesis of Ynamides
I. SYNTHESIS OF INDOLINES AND INDOLES VIA INTRAMOLECULAR [4 + 2] CYCLOADDITION OF YNAMIDES AND CONJUGATED ENYNES II. SYNTHESIS OF NITROGEN HETEROCYCLES IN SUPERCRITICAL CARBON DIOXIDE by Joshua Ross Dunetz B. A., Chemistry Haverford College, 2000 SUBMITTED TO THE DEPARTMENT OF CHEMISTRY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY September 2005 © Massachusetts Institute of Technology, 2005 All rights reserved Signature of Author .................... ·Department of Chemistry September 1, 2005 a( / Certified by ................................... ................................ Rick L. Danheiser Arthur C. Cope Professor of Chemistry, Thesis Supervisor Acceptedby......................... ............................................ I.... 7 Robert W. Field MASSACHUSETSINSTn '.vE I F TrwfhNl erv-v I Chairman, Department Committee on Graduate Students OCT 1 2005 d }cl/CF , LIBRARIES ~ This doctoral thesis has been examined by a committee in the Department of Chemistry as follows: Professor Timothy F. Jamison . ... Chairman Professor Rick L. Danheiser ........... ... ............................ Thesis Supervisor Professor Barbara Imperiali. ...... ................................................ 2 ACKNOWLEDGMENTS All acknowledgments must begin with my thesis advisor, Rick Danheiser. I first remember meeting him at the Cambridge Brewing Company during recruiting weekend five years ago, and we sat for hours in the restaurant discussing the merits of the 2000 New York Mets and whether one of our favorite baseball teams had a chance to make the playoffs that year. Ultimately, I decided to attend MIT with the hope of joining his group, and during my time in his laboratory Rick has been an excellent mentor and chemistry role model. I continue to be amazed not only by the extent of his knowledge, but also by his ability to articulate chemical principles in an easy and straightforward manner. -

Lecture 6 the Crossed Aldol Reaction and Its Many Variants
Lecture 6 The Crossed Aldol Reaction and its Many Variants Objectives: By the end of this lecture you will be able to: 1. make an appropriate choice of base to completely enolise carbonyl compounds; 2. use enolates in a crossed aldol reaction; 3. recognise the aldol functional group motif, and its variants, in complex molecules; 4. use the aldol disconnection to simplify a retrosynthetic analysis; 5. use the Reformatski and Mukaiyama aldol reactions in synthesis; 6. draw arrow-pushing mechanisms for all the aldol reactions discussed in this lecture. Introduction We have seen how choosing a base (B-) whose conjugate acid (B−H) is a much poorer acid − i.e. has a much higher pKa − than the proton we wish to abstract, ensures effectively complete deprotonation of the α-C−H of a carbonyl compound to provide the corresponding enolate. By completely enolising the carbonyl group, we can suppress self-condensation aldol processes, and instead use a different electrophile such as an aldehyde to form a β-hydroxy carbonyl compound. This reaction sequence is identical in basic mechanism to the intramolecular aldol processes that we discussed in previous lectures. It is now described as a crossed aldol condensation because the electrophile is a different carbonyl compound to the one that was used to form the nucleophilic enolate. This reaction, and its many variants, provides one of the most important methods for preparing C−C bonds. Pattern Recognition The aldol reaction and its many variants are very useful reactions in synthesis. You need to be able to identify the patterns or functional group motifs where this type of bond-forming process can be used.