Basalt Granodiorite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 1 – Introduction – Review of Rocks and Plate Tectonics Practice Exam and Study Guide
Chapter 1 – Introduction – Review of Rocks and Plate Tectonics Practice Exam and Study Guide To be able to understand the material covered during this course you need to have a basic background in the kinds of rocks making up our planet. This section of the study guide is aimed at helping you gain that background. 1. What are the three major groups of rocks found on planet Earth? Igneous Rocks 2. Which of the following processes is associated with igneous rocks? a. Solid‐state recrystallization b. Weathering and erosion c. Transportation and deposition d. Cooling a silicate liquid to a solid rock e. The accumulation of granitic debris in a moraine 3. If a silicate liquid flows out along the Earth’s surface or seabed, then it is called _______________. 4. If a silicate liquid exists beneath the Earth’s surface or seabed, then it is called _______________. 5. Which of the following terms refer to a body of magma or its solidified equivalent? a. Basalt b. Sandstone c. Gneiss d. Pluton e. Schist 6. If you can see the crystals making up an igneous rock with the naked eye, then the texture is described as a. Pyroclastic b. Phaneritic c. Aphanitic d. Porphyritic e. Aphyric from Perilous Earth: Understanding Processes Behind Natural Disasters, ver. 1.0, June, 2009 by G.H. Girty, Department of Geological Sciences, San Diego State University Page 1 7. In an aphanitic igneous rock can you make out the outlines of individual crystals with the naked eye? Yes or No 8. What type of igneous rock is the most volumetrically important on our planet? Intrusive Igneous Rocks 9. -

Oregon Geologic Digital Compilation Rules for Lithology Merge Information Entry
State of Oregon Department of Geology and Mineral Industries Vicki S. McConnell, State Geologist OREGON GEOLOGIC DIGITAL COMPILATION RULES FOR LITHOLOGY MERGE INFORMATION ENTRY G E O L O G Y F A N O D T N M I E N M E T R R A A L P I E N D D U N S O T G R E I R E S O 1937 2006 Revisions: Feburary 2, 2005 January 1, 2006 NOTICE The Oregon Department of Geology and Mineral Industries is publishing this paper because the infor- mation furthers the mission of the Department. To facilitate timely distribution of the information, this report is published as received from the authors and has not been edited to our usual standards. Oregon Department of Geology and Mineral Industries Oregon Geologic Digital Compilation Published in conformance with ORS 516.030 For copies of this publication or other information about Oregon’s geology and natural resources, contact: Nature of the Northwest Information Center 800 NE Oregon Street #5 Portland, Oregon 97232 (971) 673-1555 http://www.naturenw.org Oregon Department of Geology and Mineral Industries - Oregon Geologic Digital Compilation i RULES FOR LITHOLOGY MERGE INFORMATION ENTRY The lithology merge unit contains 5 parts, separated by periods: Major characteristic.Lithology.Layering.Crystals/Grains.Engineering Lithology Merge Unit label (Lith_Mrg_U field in GIS polygon file): major_characteristic.LITHOLOGY.Layering.Crystals/Grains.Engineering major characteristic - lower case, places the unit into a general category .LITHOLOGY - in upper case, generally the compositional/common chemical lithologic name(s) -

The Boulder Creek Batholith, Front Range, Colorado
I u The Boulder Creek Batholith, Front Range, Colorado By DOLORES J. GABLE GEOLOGICAL SURVEY PROFESSIONAL PAPER 1101 A study of differentiation, assimilation, and origin of a granodiorite batholith showing interrelated differences in chemistry and mineralogy in the batholith and cogenetic rock types UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1980 UNITED STATES DEPARTMENT OF THE INTERIOR CECIL D. ANDRUS, Secretary GEOLOGICAL SURVEY H. William Menard, Director Library of Congress Cataloging in Publication Data Gable, Dolores J. 1922- The Boulder Creek batholith, Front Range, Colorado (Geological Survey Professional Paper 1101) Bibliography: p. 85 Supt. of Docs. No.: I 19.16:1101 1. Batholiths Colorado Boulder region. I. Title. II. Series: United States Geological Survey Professional Paper 1101. QE611.5.U6G3 551.8; 8 78-24482 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 CONTENTS Page Page Abstract................................................ 1 Origin of the Boulder Creek Granodiorite and the Twin Introduction ............................................ 1 Spruce Quartz Monzonite .......................... 62 Previous work........................................... 2 Mineralogy, petrology, and chemistry of minerals in the Techniques used in this study ............................ 2 batholith.......................................... 64 Geologic setting ......................................... 3 Biotite ...'........................................... 64 The batholith .......................................... -

Geologic Map of the North Cascade Range, Washington by Ralph A
Prepared in cooperation with Washington State Division of Geology and Earth Resources, U.S. National Park Service, and U.S. Forest Service Geologic Map of the North Cascade Range, Washington By Ralph A. Haugerud and Rowland W. Tabor Nontechnical pamphlet to accompany Scientific Investigations Map 2940 Looking south from the North Klawatti Glacier [Mbse]. In the right foreground, the glacier breaks into a heavily crevassed icefall where it descends steeply. Rock in the foreground knob is Eldorado Orthogneiss (unit TKgo), a 90 million-year-old stitching pluton, which here includes numerous dikes of light- colored pegmatite. Mount Buckner on the left skyline and Mount Forbidden hidden in clouds are also eroded from the Eldorado Orthogneiss (photographed in 1987). 2009 U.S. Department of the Interior U.S. Geological Survey CONTENTS Introduction.....................................................................................................................................................1 Using this report ....................................................................................................................................1 Map preparation ...................................................................................................................................1 Major sources of new data .................................................................................................................1 Acknowledgments ................................................................................................................................2 -

Geologic Relationships of the Southern Portion of the Boston Basin from the Blue Hills Eastward
University of New Hampshire University of New Hampshire Scholars' Repository New England Intercollegiate Geological NEIGC Trips Excursion Collection 1-1-1976 Geologic Relationships of the Southern Portion of the Boston Basin from the Blue Hills Eastward Nellis, David A. Hellier, Nancy W. Follow this and additional works at: https://scholars.unh.edu/neigc_trips Recommended Citation Nellis, David A. and Hellier, Nancy W., "Geologic Relationships of the Southern Portion of the Boston Basin from the Blue Hills Eastward" (1976). NEIGC Trips. 243. https://scholars.unh.edu/neigc_trips/243 This Text is brought to you for free and open access by the New England Intercollegiate Geological Excursion Collection at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in NEIGC Trips by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. Trips A-4 & B-4 GEOLOGIC RELATIONSHIPS OF THE SOUTHERN PORTION OF THE BOSTON BASIN FROM THE BLUE HILLS EASTWARD by David A. Nellis boston State College and Nancy W. Hellier The structural, stratigraphic and petrologic relation ships in the Weymouth and Cohasset quadrangles are the princi ple subjects of this field trip. The Dedham Granodiorite is the oldest rock in this area and varies petrologically from granite to granodiorite to diorite. Overlying the Dedham Grano diorite are the lower Cambrian Weymouth Formation and the Middle Cambrian Braintree Argillite. The intrusive contact between Ordovician Quincy Granite and 3raintree Argillite will be noted. At some locations the Weymouth and Braintree formations are missing and the Dedham Granodiorite is overlain by the Bos ton Bay Group sedimentary and volcanic rocks of Pennsylvanian(?) age. -

Description of Map Units
GEOLOGIC MAP OF THE LATIR VOLCANIC FIELD AND ADJACENT AREAS, NORTHERN NEW MEXICO By Peter W. Lipman and John C. Reed, Jr. 1989 DESCRIPTION OF MAP UNITS [Ages for Tertiary igneous rocks are based on potassium-argon (K-Ar) and fission-track (F-T) determinations by H. H. Mehnert and C. W. Naeser (Lipman and others, 1986), except where otherwise noted. Dates on Proterozoic igneous rocks are uranium-lead (U-Pb) determinations on zircon by S. A. Bowring (Bowring and others, 1984, and oral commun., 1985). Volcanic and plutonic rock names are in accord with the IUGS classification system, except that a few volcanic names (such as quartz latite) are used as defined by Lipman (1975) following historic regional usage. The Tertiary igneous rocks, other than the peralkaline rhyolites associated with the Questa caldera, constitute a high-K subalkaline suite similar to those of other Tertiary volcanic fields in the southern Rocky Mountains, but the modifiers called for by some classification schemes have been dropped for brevity: thus, a unit is called andesite, rather than alkali andesite or high-K andesite. Because many units were mapped on the basis of compositional affinities, map symbols were selected to emphasize composition more than geographic identifier: thus, all andesite symbols start with Ta; all quartz latites with Tq, and so forth.] SURFICIAL DEPOSITS ds Mine dumps (Holocene)—In and adjacent to the inactive open pit operation of Union Molycorp. Consist of angular blocks and finer debris, mainly from the Sulphur Gulch pluton Qal Alluvium (Holocene)—Silt, sand, gravel, and peaty material in valley bottoms. -

Glossary of Geological Terms
GLOSSARY OF GEOLOGICAL TERMS These terms relate to prospecting and exploration, to the regional geology of Newfoundland and Labrador, and to some of the geological environments and mineral occurrences preserved in the province. Some common rocks, textures and structural terms are also defined. You may come across some of these terms when reading company assessment files, government reports or papers from journals. Underlined words in definitions are explained elsewhere in the glossary. New material will be added as needed - check back often. - A - A-HORIZON SOIL: the uppermost layer of soil also referred to as topsoil. This is the layer of mineral soil with the most organic matter accumulation and soil life. This layer is not usually selected in soil surveys. ADIT: an opening that is driven horizontally (into the side of a mountain or hill) to access a mineral deposit. AIRBORNE SURVEY: a geophysical survey done from the air by systematically crossing an area or mineral property using aircraft outfitted with a variety of sensitive instruments designed to measure variations in the earth=s magnetic, gravitational, electro-magnetic fields, and/or the radiation (Radiometric Surveys) emitted by rocks at or near the surface. These surveys detect anomalies. AIRBORNE MAGNETIC (or AEROMAG) SURVEYS: regional or local magnetic surveys that measures deviations in the earth=s magnetic field and carried out by flying a magnetometer along flight lines on a pre-determined grid pattern. The lower the aircraft and the closer the flight lines, the more sensitive is the survey and the more detail in the resultant maps. Aeromag maps produced from these surveys are important exploration tools and have played a major role in many major discoveries (e.g., the Olympic Dam deposit in Australia). -
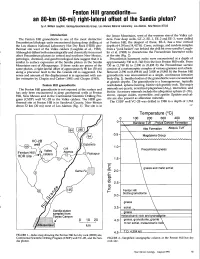
Fenton Hill Granodiorite Is One of the Most Distinctive Dera
FentonHill granodiorite- an80-km (50-mi) right-lateral otlset of the Sandia pluton? byA. WillianLaughlin, Geology/Geochemistry Group,Los Alamos National Laboratory, Los Alamos, New Mexico 87545 Introduction the Jemez Mountains, west of the western rim of the Valles cal- The Fenton Hill granodiorite is one of the most distinctive dera. Four deep wells, GT-2, EE-l, EE-2 and EE-3, were drilled Precambrianlithologic units encountered during deep drilling at at Fenton Hill; the deepest of these, EE-2, has a true vertical the Los Alamos National Laboratory Hot Dry Rock (HDR) geo- depth of 4.39km (L4,417ft). Cores,cuttings, and random samples thermal site west of the Valles caldera (Laughlin et al., 1983). from a "junk basket" run behind the drill bit were used by Laugh- Although it differs both mineralogically and chemically from most Iin et al. (1983)to characterizethe Precambrian basement rocks other Precambrianplutons in central and northern New Mexico, at the site (Fig. 2). petrologic, chemical, and geochronologicaldata suggestthat it is Precambrian basement rocks were encountered at a depth of similar to surface exposures of the Sandia pluton in the Sandia approximately 730m (I,748 ft) in the four Fenton Hill wells. From Mountains east of Albuquerque. If these rocks are pieces of the ftO m (1,748ft) to 2,590 m (8,498ft) the Precambriansection same pluton, a rightJateral offset of approximately 80 km (50 mi) consistsof a metamoryhic complexof various gneissesand schists. along a precursor fault to the Rio Grande rift is suggested.The Between 2,590 m (8,498ft) and 3,000m (9,&13ft) the Fenton Hill senseand amount of this displacementis in agreementwith ear- granodiorite was encountered as a single, continuous intrusive Iier estimatesby Chapin and Cather (1981)and Chapin (1983). -

A Partial Glossary of Spanish Geological Terms Exclusive of Most Cognates
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY A Partial Glossary of Spanish Geological Terms Exclusive of Most Cognates by Keith R. Long Open-File Report 91-0579 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. 1991 Preface In recent years, almost all countries in Latin America have adopted democratic political systems and liberal economic policies. The resulting favorable investment climate has spurred a new wave of North American investment in Latin American mineral resources and has improved cooperation between geoscience organizations on both continents. The U.S. Geological Survey (USGS) has responded to the new situation through cooperative mineral resource investigations with a number of countries in Latin America. These activities are now being coordinated by the USGS's Center for Inter-American Mineral Resource Investigations (CIMRI), recently established in Tucson, Arizona. In the course of CIMRI's work, we have found a need for a compilation of Spanish geological and mining terminology that goes beyond the few Spanish-English geological dictionaries available. Even geologists who are fluent in Spanish often encounter local terminology oijerga that is unfamiliar. These terms, which have grown out of five centuries of mining tradition in Latin America, and frequently draw on native languages, usually cannot be found in standard dictionaries. There are, of course, many geological terms which can be recognized even by geologists who speak little or no Spanish. -

Squeezing Blood from a Stone: Inferences Into the Life and Depositional Environments of the Early Archaean
Squeezing Blood From A Stone: Inferences Into The Life and Depositional Environments Of The Early Archaean Jelte Harnmeijer A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington MMX Programs Authorized to Offer Degree: Center for Astrobiology & Early Earth Evolution and Department of Earth & Space Sciences Abstract A limited, fragmentary and altered sedimentary rock record has allowed few constraints to be placed on a possible Early Archaean biosphere, likewise on attendant environmental conditions. This study reports discoveries from three Early Archaean terrains that, taken together, suggest that a diverse biosphere was already well- established by at least ~3.5 Ga, with autotrophic carbon fixation posing the most likely explanation for slightly older 3.7 - 3.8 Ga graphite. Chapter 2 aims to give a brief overview of Early Archaean geology, with special reference to the Pilbara’s Pilgangoora Belt, and biogeochemical cycling, with special reference to banded-iron formation. Chapter 3 reports on the modelled behaviour of abiotic carbon in geological systems, where it is concluded that fractionations incurred through autotrophic biosynthesis are generally out of the reach of equilibrium processes in the crust. In Chapter 4, geological and geochemical arguments are used to identify a mixed provenance for a recently discovered 3.7 - 3.8 Ga graphite-bearing meta-turbidite succession from the Isua Supracrustal Belt in southwest Greenland. In Chapter 5 and 6, similar tools are used to examine a newly discovered 3.52 Ga kerogenous and variably dolomitized magnetite-calcite meta- sediment from the Coonterunah Subgroup at the base of the Pilbara Supergroup in northwest Australia. -

Geology and Mineral Resources of Dwyer Quadrangle, Grant, Luna, and Sierra Counties, New Mexico
BULLETIN 38 Geology and Mineral Resources of Dwyer Quadrangle, Grant, Luna, and Sierra Counties, New Mexico by WOLFGANG E. ELSTON 1957 STATE BUREAU OF MINES AND MINERAL RESOURCES NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY CAMPUS STATION SOCORRO, NEW MEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS MEMBERS Ex OFFICIO The Honorable Edwin L. Mechem....................... Governor of New Mexico Mrs. Georgia L. Lusk .........................Superintendent of Public Instruction APPOINTED MEMBERS Robert W. Botts ...................................................................... Albuquerque Holm 0. Bursum, Jr. ........................................................................ Socorro Thomas M. Cramer ........................................................................ Carlsbad John N. Mathews, Jr. ...................................................................... Socorro Richard A. Matuszeski ............................................................ Albuquerque Contents Page ABSTRACT ........................................................................................................ i INTRODUCTION ......................................................................................... 1 Purpose and scope ....................................................................................... 1 Methods of investigation ............................................................................. 1 Previous work -

The Nature and Polygenetic Origin of Orbicular Granodiorite in the Lower Castle Creek Pluton, Northern Sierra Nevada Batholith, California
Origin and Evolution of the Sierra Nevada and Walker Lane themed issue 00644 1st pages / page 1 of 9 The nature and polygenetic origin of orbicular granodiorite in the Lower Castle Creek pluton, northern Sierra Nevada batholith, California Arthur Gibbs Sylvester Department of Earth Science, University of California, Santa Barbara, California 93106, USA ABSTRACT batholith (Moore and Lockwood, 1973), but of those elsewhere in the Sierra Nevada (Moore also in plutons worldwide, especially in Scan- and Lockwood, 1973), although they are larger Mafi c and granodioritic magmas mingled dinavia (e.g., Sederholm, 1928; Simonen, 1966; and better developed than most Sierran exam- to produce a swarm of microdiorite enclaves Lahti, 2005). The orbicules themselves may ples (Fig. 2). Large mafi c enclaves with felsic in the granodiorite. The enclaves and rare have cores of other kinds of rocks besides mafi c haloes have also been noted but not described by hornfels inclusions were carried upward in an rocks, including metamorphic rock fragments Tobisch et al. (1997, their fi g. 8c, p. 333) in the oval-shaped pipe, 30 m long and 15 m wide, and single crystals or clots of minerals such as central Sierra Nevada batholith. probably as a gas-driven mass to a point where K-feldspar megacrysts (e.g., Lahti, 2005). an H2O-rich, superheated felsic melt intruded This paper describes an assemblage and ori- TERMINOLOGY the pipe and the enclave mass, and upon gin of some remarkable orbicular rocks with abrupt undercooling, deposited orbicu lar felsic haloes in a single pipe-like exposure in Mafi c magmatic enclaves (MME in the ter- shells of tangentially oriented micro crystals the granodiorite of Lower Castle Creek pluton minology of Barbarin, 2005; also autoliths of sodic plagioclase, quartz, K-feldspar , and in the northern Sierra Nevada batholith (Fig.