Survey of Pathogens and Parasitoids in Late Instar Lymantria Dispar Larval Populations in Sardinia, Italy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comune Di Orune Provincia Di Nuoro Ufficio Personale °°°°°°
COMUNE DI ORUNE PROVINCIA DI NUORO UFFICIO PERSONALE °°°°°° DETERMINAZIONE N. 694 DEL 28.11.2017 OGGETTO: Concorso pubblico per esami per copertura a tempo part-time al 75% e indeterminato di n. 1 “Istruttore Amministrativo” Categoria C – posizione economica C1. Ammissione o esclusione candidati. ========= IL RESPONSABILE DEL SERVIZIO DATO atto che sulla GURI , 4^ Serie Speciale – Concorsi ed Esami n. 81 del 24.10-2017, veniva pubblicato un bando di concorso pubblico per soli esami per l’assunzione, a tempo part-time al 70% ed indeterminato, di n. 1 “Istruttore Amministrativo”, Categoria C, posizione economica C1, da destinare al settore amministrativo, in esecuzione della propria determinazione n. 543 del 29.09.2017 di approvazione del bando medesimo; ATTESO che ai sensi del vigente Regolamento degli uffici e servizi e delle norme dell’accesso agli impieghi vigente in questo Comune, è compito del sottoscritto l’esame delle domande presentate dai concorrenti ai fini dell’ammissione o esclusione dalle prove; VISTO a tal fine il già citato regolamento comunale per l’accesso agli impieghi e il dettato del bando stesso che costituisce ad ogni effetto interpretativo “Lex Specialis” del concorso stesso; ESAMINATE le domande presentate, alla luce di quanto sopra espresso; DETERMINA Di AMMETTERE alle prove concorsuali i seguenti candidati: N° NOMINATIVO LUOGO E DATA DI NASCITA COMUNE DI RESIDENZA 1 ZIDDA SONIA NUORO 03.06.1988 ORUNE 2 ZIDDA ANNALISA NUORO 15.05.1994 ORUNE 3 ZANZA ANTONELLA SASSARI 30.01.1988 BOSA 4 VINZI AGNESE NUORO 10.10.1981 OLIENA 5 VIAGGIU ANDREA SARDARA 21.07.1972 SARDARA 6 VARGIU PIERO IGLESIAS 25.08.1982 S. -

Corso Di Formazione Per Operatore Socio Sanitario
UNIONE EUROPEA PROVINCIA DI NUORO Fondo Sociale Europeo Assessorato del Lavoro, Formazione Professionale, Cooperazione e Sicurezza sociale SETTORE LAVORO, FORMAZIONE PROFESSIONALE E POLITICHE SOCIALI AVVISO PUBBLICO PER L’ATTUAZIONE DEL PIANO PROVINCIALE DI FORMAZIONE PROFESSIONALE ANNUALITÀ 2011-2012 CIG. 4810202044 CORSO DI FORMAZIONE PER OPERATORE SOCIO SANITARIO GRADUATORIA RESIDENTI IN PROVINCIA DI NUORO IN ORDINE DI PUNTEGGIO Sono evidenziati in verde gli ammessi alla prova orale Prova 1 Prova 2 Titoli Titoli Valutazione Test Posizione Cognome Nome Data di nascita Luogo residenza Prov residenza Esperienza disoccupazione TOTALE competenze psicoattitudinale (MAX 17 Punti) (MAX 8 punti) (MAX 25 punti) (MAX 30 punti) 1 SANNA MARIA FRANCESCA 08/11/1972 MEANA SARDO NUORO 4,8 4 20 28,23 57,03 2 SALIS PIETRINA 22/03/1978 OLIENA NUORO 5 8 21 22,64 56,64 3 TIDU BARBARA 23/09/1971 TETI NUORO 12,2 8 23 12,33 55,53 4 CURRELI GIUSEPPINA 16/11/1977 ARITZO NUORO 13 1,5 25 15,58 55,08 5 SEDDA MARTA 13/08/1984 OLLOLAI NUORO 12 1,5 25 15,49 53,99 6 CONGIU GIOVANNA MARIA 08/12/1973 SILANUS NUORO 4,8 8 25 15,80 53,60 7 LICHERI BARBARA 29/12/1977 OLZAI NUORO 9 0 25 19,31 53,31 8 SECHI JENNIFER MARIA LUISA 16/11/1980 BORORE NUORO 7,8 8 25 12,34 53,14 9 DELIGIA SALVATORANGELA 29/06/1981 BORORE NUORO 12,4 1,5 25 14,18 53,08 10 MORO ANTONIETTA 30/09/1966 OLZAI NUORO 10 8 23 12,01 53,01 11 MELONI RITA 05/09/1966 ORTUERI NUORO 15,2 8 15 14,68 52,88 12 CARBONI FRANCESCA 31/10/1969 TONARA NUORO 17 8 12 15,64 52,64 13 TALLORU SERGIO MAURO 26/05/1985 TONARA NUORO -

GIS-Based Landscape Analysis of Megalithic Graves in the Island of Sardinia (Italy) Riccardo Cicilloni 1, Marco Cabras 2
GIS-based landscape analysis of megalithic graves in the Island of Sardinia (Italy) Riccardo Cicilloni 1, Marco Cabras 2 1. Department of History, Cultural Heritage and Territory, University of Cagliari. Via Is Mirrionis 1, 09123 Cagliari, Italy. Email: [email protected] 2. Ph.D. Candidate, Doctorado en Historia y Artes – Arquelogía y Cultura Material, Universidad de Granada. Via Is Mirrionis 119, 09121 Cagliari, Italy. Email: [email protected] Abstract: One of the most important megalithic groups in Western Europe in terms of number and characteristics is the group of over 200 monuments of various types in Sardinia. It now seems to be confirmed that the rise of the megalithic phenomenon was during the culture of San Michele of Ozieri (Late Neolithic, 4000-3300 B.C.E.). The Sardinian dolmen graves, however, had a maximum distribution during the Chalcolithic, as evidenced by most of the finds from excavations. The phenomenon also shows a close relationship beyond Sardinia and especially with the monuments of Catalonia, Pyrenees, non-coastal departments of French-midi, Corsica and Puglia. About 90 dolmen graves of various types have been investigated, namely the simple type, “corridor” type, “allée couverte” type, and others of uncertain attribution, located in central-western Sardinia, and particularly in a significant area of ca. 3500 km2 coinciding with the historical regions of Marghine-Planargia, Middle Valley of Tirso and Montiferru. This includes some 40% of all Sardinian dolmens. Locational trends and relationships with regard to landscape elements were studied with the aid of GIS methodologies such as viewshed and cost surface analysis. -

Determinazione ATS N.6235 Del 07/08/2019
SERVIZIO SANITARIO REGIONE AUTONOMA DELLA SARDEGNA ATS DETERMINAZIONE DIRIGENZIALE N° ____ DEL __/__/____ Proposta n. 6166 del 15/07/2019 STRUTTURA PROPONENTE: SC RICERCA E SELEZIONE DELLE RISORSE UMANE Dr.ssa Patrizia Sollai OGGETTO: Convenzione tra l'ATS Sardegna ASSL Nuoro e il Servizio Politiche per l'impresa dell'Assessorato Regionale del Lavoro, Formazione Professionale, Cooperazione e Sicurezza Sociale finalizzata alla prosecuzione nel triennio 2019/2021 dei cantieri occupazionali ai sensi dell'art. 5 comma 13 L.R. N. 5/2015 con integrazioni introdotte dalla L.22/2017 art. 1 comma 7, in attuazione dell'art. 8 comma 45 LR n. 48/2018. Avvio dei progetti presso l'ASSL di Nuoro. Con la presente sottoscrizione i soggetti coinvolti nell'attività istruttoria, ciascuno per le attività e responsabilità di competenza dichiarano che la stessa è corretta, completa nonché conforme alle risultanze degli atti d'ufficio, per l'utilità e opportunità degli obiettivi aziendali e per l'interesse pubblico. RUOLO SOGGETTO FIRMA DIGITALE Estensore Dr.ssa Lucia Maria Cadeddu Responsabile del procedimento La presente Determinazione prevede un impegno di spesa a carico della Azienda per la Tutela della Salute SI [ ] NO [ X] DA ASSUMERE CON SUCCESSIVO PROVVEDIMENTO [ ] La presente Determinazione è soggetta al controllo preventivo di cui al comma 1 dell’art. 29 della L.R. 10/2006 e ss.mm.ii. SI [ ] NO [ X] Pagina 1 di 6 IL DIRETTORE DELLA SC RICERCA E SELEZIONE DELLE RISORSE UMANE VISTA la Deliberazione del Direttore Generale n. 204 del 09/02/2018 con la quale è stato attribuito alla Dr.ssa Patrizia Sollai l’incarico di Direttore della S.C. -

Mattu Antonina
F ORMATO EUROPEO PER IL CURRICULUM VITAE INFORMAZIONI PERSONALI Nome MATTU ANTONINA Qualifica SEGRETARIO GENERALE Amministrazione COMUNE DI LANUSEI (NU) Telefono 0782/473130 E-mail [email protected] - ([email protected]) Nazionalità Italiana Data di nascita OMISSIS … ESPERIENZA LAVORATIVA DA DICEMBRE 2016 AD OGGI SEGRETARIO GENERALE DELLA CONVENZIONE TRA IL COMUNE DI LANUSEI E I COMUNI DI OLZAI E OLLOLAI OLLOLAI (NU) • INCARICO DI PRESIDENTE NUCLEO VALUTAZIONE • INCARICO DI PRESIDENTE DELLA DELEGAZIONE TRATTANTE DI PARTE PUBBLICA • INCARICO DI PRESIDENTE DELLA COMMISSIONE PER I PROCEDIMENTI DISCIPLINARI • SOSTITUZIONE RESPONSABILI PP.OO.. • RESPONSABILE ANTICORRUZIONE E TRASPARENZA DA OTTOBRE 2005 AD AGOSTO SEGRETARIO GENERALE – SEDE TITOLARE COMUNE SINISCOLA .(NU) 2016 • NOMINA DI DIRETTORE (FINO AL MAGGIO 2016) • INCARICO DI PRESIDENTE NUCLEO VALUTAZIONE , DELLA DELEGAZIONE TRATTANTE • RESPONSABILE UFFICIO PER I PROCEDIMENTI DISCIPLINARI • SOSTITUZIONE RESPONSABILI LE PP.OO. IN CASO DI ASSENZA. • RESPONSABILE ANTICORRUZIONE (DAL 24/01/2014) • SUPPLENZE A SCAVALCO PRESSO I COMUNI DI BITTI, LOCULI, OLZAI E OLLOLAI (NU) DAL GENNAIO 2001 FINO A TITOLARE SEGRETERIA CONSORZIATA DEI COMUNI DI TONARA DESULO (NU) SETTEMBRE 2005 SUPPLENZE A SCAVALCO PRESSO I COMUNI DI ARITZO, BELVÌ, GADONI(NU) DAL MAGGIO 1997 FINO AL GENNAIO SEGRETARIO COMUNALE – SEDE TITOLARE COMUNE TONARA.(NU) 2001 • INCARICO DI PRESIDENTE NUCLEO VALUTAZIONE, • PRESIDENTE DELLA DELEGAZIONE TRATTANTE • RESP SERVIZIO AMM.VO E SOSTITUZIONE RESPONSABILI DI SERVIZIO IN CASO DI ASSENZA. • SUPPLENZE A SCAVALCO PRESSO I COMUNI DI ARITZO, BELVÌ E DESULO(NU) DAL FEBBRAIO AL MAGGIO 1997 SEGRETARIO COMUNALE – SEDE TITOLARE COMUNE DI OVODDA (NU) DAL 1990 AL 1996 SEGRETARIO COMUNALE – SEDE TITOLARE COMUNE DI TIANA.(NU) SUPPLENZE A SCAVALCO PRESSO I COMUNI DI ARITZO, AUSTIS, BELVÌ, GAVOI, MAMOIADA, TONARA E TETI. -

Curriculum Vitae Maria Paola Dettori
Curriculum Vitae Maria Paola Dettori Informazioni personali Cognome / Nome Dettori Maria Paola Telefono 0792067407 E-mail [email protected] Cittadinanza Italiana Maria Paola Dettori si laurea in Storia dell’Arte presso l’Università degli Studi di Cagliari, dove poi prosegue con la Scuola di specializzazione in Storia dell’Arte. Master di II livello “DECApro - Master Interdipartimentale in Diritto ed Economia per la Cultura e l’Arte nella Progettazione dello sviluppo territoriale”, Università degli Studi di Sassari Nel 2001 consegue l’idoneità al concorso per Funzionario Storico dell’Arte e Storico dell’Arte Direttore nei ruoli del Ministero dei Beni Culturali – Regione Sardegna. Di ruolo nella PA dal 2000, in servizio presso il MiBACT dall’ottobre 2005. Lavoro o posizione Funzionario Storico dell’arte Responsabile Area funzionale Patrimonio storico artistico ricoperti della Soprintendenza Archeologia Belle Arti e Paesaggio per le Province di Sassari e Nuoro / Incarichi di tutela, conservazione e valorizzazione del patrimonio culturale/ già Direttore Pinacoteca Nazionale di Sassari Principali attività e Responsabile Area funzionale Patrimonio storico artistico - Direttore Ufficio responsabilità attuali Esportazione opere d’arte contemporanea – Responsabile Ufficio Catalogo – Progettista e direttore Lavori di restauro - Curatrice mostre - Autrice di pubblicazioni scientifiche Incarichi e attività Incarichi e attività per il MiBACT 2019 (in corso) Responsabile di Area per il patrimonio storico artistico Direttore dell’Ufficio Esportazione Opere d’arte contemporanea Responsabile Ufficio Mostre e prestiti opere d’arte Progettista e Direttore lavori restauro opere d’arte di: Benetutti e Bortigali, Retabli Maestro di Ozieri, dipinti sec. XVI; Parrocchia sant’Antioco martire di Atzara (NU), statue lignee secc. XVII-XVIII; Chiesa santa Trinità di Sassari, dipinti secc. -

Presentazione Primavera Nel Marghine, Ogliastra E Baronia 2016
Primavera nel Marghine, Ogliastra e Baronia Edizione 2016 Comunicato stampa Nuoro, 13 aprile. Dal 16 aprile sino al 26 giugno 2016 si rinnova il consueto appuntamento primaverile che dal 2006 propone il viaggio tra i borghi e i paesi del Cuore della Sardegna. Alla conferenza stampa di presentazione della decima edizione della Primavera nel Marghine, Ogliastra e Baronia hanno partecipato il Presidente della CCIAA di Nuoro Agostino Cicalò, dell’Aspen Vincenzo Cannas e i rappresentanti di alcuni dei comuni aderenti al circuito (Lotzorai, Lodè, Posada, Urzulei, Bortigali, Bari Sardo, Arbatax – Tortolì, Girasole, Loceri). La Primavera è un lungo percorso che vedrà protagonisti venti paesi dalle affascinanti bellezze naturali e paesaggistiche, antiche tradizioni e un ricco patrimonio enogastronomico. Nel calendario della Primavera si confermano alcuni paesi dello scorso anno, come Girasole, Lodè, Siniscola, Bosa, Triei, Lotzorai, Macomer, Baunei, Arbatax, Bari Sardo, Urzulei e Lanusei che metteranno in luce le particolarità e le eccellenze dei diversi territori. Tra le novità dell’edizione 2016 si segnala la partecipazione di Loceri, Ilbono, Sindia, Posada, Lei, Osini, Bortigali e Birori. Il Viaggio nel Cuore della Sardegna in Primavera propone soprattutto la scoperta dei paesaggi incantati e della natura incontaminata, tra i colori intensi della natura e la costa dalle numerose varietà del turchese. “Fino a quando l’Aspen e la Camera di Commercio potranno proseguire nelle proprie attività - dichiara il Presidente Cannas - il nostro impegno sarà quello di continuare ad investire nelle manifestazioni utili per allungare la stagione turistica. Tutto ciò è possibile grazie al coordinamento con le amministrazioni comunali e con le realtà locali che vantano una particolare bravura nella realizzazione di prodotti di artigianato e dell’enogastronomia”. -
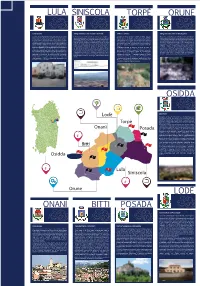
Bitti Posada Lodè Osidda Orune Torpè Siniscola Lula
LULA SINISCOLA TORPÈ ORUNE Casa Museo Adeguamento Casa Tancale Carzedda Ufficio Turistico Adeguamento aree archeologiche. Il comune di Lula intende intervenire sulle strutture della Casa L’edificio Casa Carzedda-Tancale destinato a casa del Parco, La casa del Parco presso il comune di Torpè oggetto Orune Area archeologica di Sant’Efis. Il sito archeologico Museo, sito in Centro storico, nella via retrostante Piazza situato nel centro storico di Siniscola in Via Piemonte n°20 da dell’intervento, si sviluppa su due piani con superficie risalente al periodo romano, nel territorio comunale di Orune. Costituzione, sulla quale si affaccia l’edificio adibito a Centro destinarsi a Casa del Parco, è stato recuperato a valere sui fondi coperta pari a m 50 al piano terra e mq 80 al primo piano. Nel sito è stato identificato un abitato di età romana imperiale di Educazione Ambientale, anch’esso di proprietà comunale e CIVIS –Rafforzamento centri minori. La casa, grazie al presente L’amministrazione comunale intende mettere in funzione la assai esteso nel corso di scavi archeologici realizzati a cura contiguo e comunicante con essa. Con l’intervento proposto si intervento dovrà ospitare uno spazio espositivo permanente casa del parco come Ufficio Turistico della rete dei parchi della Soprintendenza Archeologica di Nuoro nel 1992/93 e nel intende allestire la Casa Museo per farne un vero e proprio un relativo alla cultura del territorio, la struttura gestionale del delle Baronie, punto di riferimento per l’intero percorso in 2002/03. L’intervento che si intende realizzare è finalizzato al pit stop all’interno dell’itinerario turistico, prevedendo anche Parco ed un centro di documentazione multimediale rivolto a termini di supporto, accoglienza e accompagnamento del miglioramento della accessibilità e dunque della capacità di allestire spazi appositi per lo svolgimento delle attività di turisti, semplici cittadini ed imprese. -

Filoloxía Asturiana
REVISTA DE Filoloxía Asturiana volume 11/12 - años 2011/2012 Revista de Filoloxía Asturiana Revista de Filoloxía Asturiana (Anuariu universitariu d’estudios llingüísticos y lliterarios asturianos y románicos) Edita: Grupu d’Investigación Seminariu de Filoloxía Asturiana Universidá d’Uviéu Director: Xulio Viejo Fernández Secretaria: Taresa Fernández Lorences Comité de Redacción Fernando Álvarez-Balbuena García (Dptu. Filoloxía Clásica y Románica, Universidá d’Uviéu), Ramón d’Andrés Díaz (Dptu. Filoloxía Española, Universidá d’Uviéu), Xuan Carlos Busto Cortina (Dptu. Filoloxía Clásica y Románica, Universidá d’Uviéu), María Cueto Fernández (Dptu. Filoloxía Española, Universidá d’Uviéu), Iván Cuevas, Taresa Fernández Lorences (Dptu. Filoloxía Española, Universidá d’Uviéu), Roberto Hinojal Díaz (Dptu. Filoloxía Española, Universidá d’Uviéu), Rosa María Medina Granda (Dptu. Filoloxía Clásica y Románica, Universidá d’Uviéu), Leopoldo Sánchez Torre (Dptu. Filoloxía Española, Universidá d’Uviéu), Xulio Viejo Fernández (Dptu. Filoloxía Española, Universidá d’Uviéu) Miembros del Seminariu de Filoloxía Asturiana de la Universidá d’Uviéu Comité Científicu Rosario Álvarez (Universidade de Santiago de Compostela-Instituto da Lingua Galega), Antonio Bárbolo Alves (CEL-Universidade de Trás-os-Mon- tes e Alto Douro, Portugal), Eduardo Blasco Ferrer (Università di Cagliari, Cerdeña), Inés Fernández Ordóñez (Universidad Autónoma de Madrid-Real Academia Española), José Enrique Gargallo Gil (Universitat de Barcelona), Hans Goebl (Universität Salzburg, Austria), -

Proposta Di Carta Nazionale Delle Aree
Magomadas Flussio Birori Bortigali Silanus Bolotana Orani Macomer Oliena Dualchi Noragugume Tresnuraghes Dorgali Scano di Sennariolo Ottana Sarule Montiferro Borore Mamoiada Sedilo Olzai Ollolai ¤ Aidomaggiore Orgosolo Norbello Cuglieri Santu Lussurgiu Sorradile Lodine TAV. 2 Abbasanta Soddì Gavoi Bidonì Urzulei Tadasuni Teti Nughedu Santa Baunei Boroneddu Vittoria PROPOSTA DI Ovodda Bonarcado Seneghe Ghilarza Fonni Ardauli TAV. 3 CARTA NAZIONALE DELLE Austis Tiana Neoneli AREE POTENZIALMENTE IDONEE Paulilatino Ulà Tirso Narbolia Milis (ex art. 27 D.Lgs. 31/2010 e ss. mm. ii.) Talana TAV. 4 Villagrande San Vero Bauladu Sorgono Ortueri Tonara Strisaili Milis Triei Busachi Desulo TAV. 5 Riola Sardo Tramatza Villanova Baratili Truschedu Fordongianus San Pietro Zeddiani Atzara Lotzorai Zerfaliu Solarussa Nurachi Belvì Ollastra TAV. 6 Siamaggiore Girasole Allai Samugheo Arzana TAV. 1 Aritzo Siapiccia OR-58 Meana Sardo Cabras Simaxis Quadro d'unione delle tavole Oristano Tortolì Siamanna Ruinas Elini Gadoni Ilbono Seulo Villa Gairo Villaurbana Asuni Mogorella Sant'Antonio Lanusei Seui Palmas Laconi Tavola 5 - Sardegna Santa Giusta Arborea OR-60 Loceri Bari Sardo Osini Usellus Villa Verde OR-59 Ussassai Senis Nureci Sadali Nurallao Pau OR-61 Assolo Genoni Villanova Albagiara Nuragus Tulo Marrubiu Arborea Ales Jerzu Cardedu Gonnosnò SU-49 Isili Morgongiori Sini Curcuris Ulassai Genuri Tuili SU-47 Gesturi Esterzili Pompu Simala Baressa Baradili Siris Setzu Terralba Serri SU-45 Nurri Uras Ubicazione Area Potenzialmente Idonea* Masullas SU-65 Turri -

RISORSE IDROGEOLOGICHE E NATURALISTICHE Pag
1 SOMMARIO 1. Il TERRITORIO COMUNALE Pag. 04 1.1. DATI TERRITORIALI DI CARATTERE GENERALE Pag. 04 1.2. INQUADRAMENTO TERRITORIALE Pag. 04 1.3. RISORSE IDROGEOLOGICHE E NATURALISTICHE Pag. 04 2. ANALISI AMBIENTALE E GEOLOGICA DEL TERRITORIO Pag. 06 2.1. PATRIMONIO AGRICOLO E FORESTALE Pag. 06 2.2. FORMAZIONE GEOLOGICA Pag. 06 3. ANALISI STORICO-CULTURALE Pag. 08 3.1. ORIGINI STORICHE DEL PAESE Pag. 08 3.2. I BENI ARCHITETTONICI Pag. 08 3.2.1 La Chiesa di S.Gavino Pag. 08 3.2.2 La Chiesa di S.Antioco Pag. 09 3.2.3 La Chiesa del Carmelo Pag. 09 3.2.4 La Chiesa di S.Giovanni Battista Pag. 09 3.2.5 Il Santuario della madonna d'Itria Pag. 10 3.3. I BENI ARCHEOLOGICI Pag. 10 4. ANALISI DEMOGRAFICA Pag. 12 4.1. POPOLAZIONE RESIDENTE Pag. 12 4.2. DISTRIBUZIONE DELLA POPOLAZIONE SUL TERRITORIO Pag. 18 4.3. POPOLAZIONE SCOLASTICA Pag. 20 4.4. STRUTTURA PER ETA' DELLA POPOLAZIONE Pag. 22 4.5. STRUTTURA FAMILIARE E ANDAMENTO DEL NUMERO DELLE Pag. 24 FAMIGLIE 4.6. POPOLAZIONE STRANIERA RESIDENTE Pag. 25 5. ANALISI SOCIO ECONOMICA Pag. 28 5.1. IL CONTESTO ECONOMICO E IL TESSUTO PRODUTTIVO Pag. 28 5.2. ATTIVITA' PRODUTTIVA E TENDENZA IN ATTO Pag. 28 6. ANALISI ASSETTO INSEDIATIVO Pag. 32 6.1. EDIFICATO URBANO Pag. 32 6.2. UNITA’ EDILIZIE ESISTENTI E LORO UTILIZZO Pag. 33 6.3. DATA DI COSTRUZIONE Pag. 34 6.4. EDIFICATO ZONA AGRICOLA Pag. 34 6.5. INSEDIAMENTI TURISTICI SUL LAGO Pag. 35 6.6. -

Nome Graziella Mellino Residenza Nule – SS Telefono +39.3496210273 E-Mail [email protected] Nazionalità Italiana Data Di Nascita 17/1/1967
CURRICULUM VITAE INFORMAZIONI PERSONALI Nome Graziella Mellino Residenza Nule – SS Telefono +39.3496210273 E-mail [email protected] Nazionalità Italiana Data di nascita 17/1/1967 ESPERIENZE LAVORATIVE • Date Da settembre 2008 ad oggi • Nome e indirizzo del datore Dasein S.r.l - Sardegna di lavoro Lungo Dora Colletta, 81 – Torino Sede locale: Oristano – Via Liguria, 22 • Tipo di azienda o settore Consulenza – Ricerca – Formazione Enti Locali • Tipo di impiego Lavoro dipendente a tempo indeterminato Principali Mansioni Consulente esperto nella gestione delle risorse umane , in particolare: • Gestione di sistemi di valutazione ai sensi dei Contratti Collettivi Nazionali di Lavoro Enti Locali , in particolare riferiti alle posizioni organizzative (valutazione della posizione e del risultato) ed ai sistemi permanenti di valutazione ex art. 6, relativi alle prestazioni e alle progressioni del personale dell'Ente Locale • Attuale componente dei Nuclei di Valutazione nei seguenti comuni: Aritzo, Atzara, Baunei, Belvi, Bitti, Bolotana, Borore, Bortigali, Cardedu, Comunità Montana Mandrolisai, Consorzio Bimf, Flussio, Irgoli, Gadoni, Gairo, Gavoi, Jerzu, Lei, Lodè, Laconi, Lula, Magomadas, Mamoiada, Meana Sardo, Noragugume, Ollolai, Olzai, Onani, Orani, Ortueri, Orune, Osidda, Osini, Ovodda, Posada, Perdasdefogu, Sagama, San Teodoro, Sarule, Scano di Montiferro, Sennariolo, Seui, Sorgono, Talana, Tiana, Tinnura, Tonara, Torpè, Ulassai, Unione dei Comuni del Montalbo, Unione dei Comuni della Barbagia, Unione dei Comuni della Valle del Pardu e dei Tacchi, Urzulei, Ussassai, Villagrande Strisaili. Valutazione, esercizio 2012, ai sensi dell’art. 1 commi 39-40 della L. 190/2012, sulle motivazioni addotte dagli enti a giustificazione delle anomalie riscontrate nell’ambito della rilevazione sulle tipologie di lavoro flessibile di cui all’art.