Remarkably High Rate of DNA Amplification Promoted by the Mating-Type Switching Mechanism in Schizosaccharomyces Pombe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Download
Supplementary Figure S1. Results of flow cytometry analysis, performed to estimate CD34 positivity, after immunomagnetic separation in two different experiments. As monoclonal antibody for labeling the sample, the fluorescein isothiocyanate (FITC)- conjugated mouse anti-human CD34 MoAb (Mylteni) was used. Briefly, cell samples were incubated in the presence of the indicated MoAbs, at the proper dilution, in PBS containing 5% FCS and 1% Fc receptor (FcR) blocking reagent (Miltenyi) for 30 min at 4 C. Cells were then washed twice, resuspended with PBS and analyzed by a Coulter Epics XL (Coulter Electronics Inc., Hialeah, FL, USA) flow cytometer. only use Non-commercial 1 Supplementary Table S1. Complete list of the datasets used in this study and their sources. GEO Total samples Geo selected GEO accession of used Platform Reference series in series samples samples GSM142565 GSM142566 GSM142567 GSM142568 GSE6146 HG-U133A 14 8 - GSM142569 GSM142571 GSM142572 GSM142574 GSM51391 GSM51392 GSE2666 HG-U133A 36 4 1 GSM51393 GSM51394 only GSM321583 GSE12803 HG-U133A 20 3 GSM321584 2 GSM321585 use Promyelocytes_1 Promyelocytes_2 Promyelocytes_3 Promyelocytes_4 HG-U133A 8 8 3 GSE64282 Promyelocytes_5 Promyelocytes_6 Promyelocytes_7 Promyelocytes_8 Non-commercial 2 Supplementary Table S2. Chromosomal regions up-regulated in CD34+ samples as identified by the LAP procedure with the two-class statistics coded in the PREDA R package and an FDR threshold of 0.5. Functional enrichment analysis has been performed using DAVID (http://david.abcc.ncifcrf.gov/) -

Association of Gene Ontology Categories with Decay Rate for Hepg2 Experiments These Tables Show Details for All Gene Ontology Categories
Supplementary Table 1: Association of Gene Ontology Categories with Decay Rate for HepG2 Experiments These tables show details for all Gene Ontology categories. Inferences for manual classification scheme shown at the bottom. Those categories used in Figure 1A are highlighted in bold. Standard Deviations are shown in parentheses. P-values less than 1E-20 are indicated with a "0". Rate r (hour^-1) Half-life < 2hr. Decay % GO Number Category Name Probe Sets Group Non-Group Distribution p-value In-Group Non-Group Representation p-value GO:0006350 transcription 1523 0.221 (0.009) 0.127 (0.002) FASTER 0 13.1 (0.4) 4.5 (0.1) OVER 0 GO:0006351 transcription, DNA-dependent 1498 0.220 (0.009) 0.127 (0.002) FASTER 0 13.0 (0.4) 4.5 (0.1) OVER 0 GO:0006355 regulation of transcription, DNA-dependent 1163 0.230 (0.011) 0.128 (0.002) FASTER 5.00E-21 14.2 (0.5) 4.6 (0.1) OVER 0 GO:0006366 transcription from Pol II promoter 845 0.225 (0.012) 0.130 (0.002) FASTER 1.88E-14 13.0 (0.5) 4.8 (0.1) OVER 0 GO:0006139 nucleobase, nucleoside, nucleotide and nucleic acid metabolism3004 0.173 (0.006) 0.127 (0.002) FASTER 1.28E-12 8.4 (0.2) 4.5 (0.1) OVER 0 GO:0006357 regulation of transcription from Pol II promoter 487 0.231 (0.016) 0.132 (0.002) FASTER 6.05E-10 13.5 (0.6) 4.9 (0.1) OVER 0 GO:0008283 cell proliferation 625 0.189 (0.014) 0.132 (0.002) FASTER 1.95E-05 10.1 (0.6) 5.0 (0.1) OVER 1.50E-20 GO:0006513 monoubiquitination 36 0.305 (0.049) 0.134 (0.002) FASTER 2.69E-04 25.4 (4.4) 5.1 (0.1) OVER 2.04E-06 GO:0007050 cell cycle arrest 57 0.311 (0.054) 0.133 (0.002) -

Gene Products of Chromosome 11Q and Their Association with CCND1
Available online http://breast-cancer-research.com/content/10/5/R81 ResearchVol 10 No 5 article Open Access Gene products of chromosome 11q and their association with CCND1 gene amplification and tamoxifen resistance in premenopausal breast cancer Katja Lundgren1, Karolina Holm2, Bo Nordenskjöld3,4, Åke Borg2 and Göran Landberg1 1Center for Molecular Pathology, Department of Laboratory Medicine, Lund University, Malmö University Hospital, Malmö, SE-205 02, Sweden 2Department of Clinical Sciences, Division of Oncology, Lund University, Lund, SE-221 85, Sweden 3Department of Oncology, Borås Hospital, Borås, SE-501 82, Sweden 4Division of Oncology, Faculty of Health Sciences, Linköping University, Linköping, SE-581 85, Sweden Corresponding author: Göran Landberg, [email protected] Received: 2 Apr 2008 Revisions requested: 8 May 2008 Revisions received: 4 Aug 2008 Accepted: 29 Sep 2008 Published: 29 Sep 2008 Breast Cancer Research 2008, 10:R81 (doi:10.1186/bcr2150) This article is online at: http://breast-cancer-research.com/content/10/5/R81 © 2008 Lundgren et al.; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Introduction The amplification event occurring at chromosome protein expression was also compared with gene expression locus 11q13, reported in several different cancers, includes a data in a subset of 56 breast cancer samples. number of potential oncogenes. We have previously reported amplification of one such oncogene, namely CCND1, to be correlated with an adverse effect of tamoxifen in premenopausal Results Cortactin and FADD (Fas-associated death domain) breast cancer patients. -

Rin1 Is a Negative Regulator of the IL3 Receptor Signal Transduction Pathways
ANTICANCER RESEARCH 26: 905-916 (2006) Rin1 is a Negative Regulator of the IL3 Receptor Signal Transduction Pathways C.M. HUNKER, A. GALVIS, M.L. VEISAGA and M.A. BARBIERI Department of Biological Sciences, Florida International University, University Park, Miami, Florida 33199, U.S.A. Abstract. Cytokines interact with cell-surface receptors, initiating (9:22 translocation) is often found in patients with leukemia, signaling cascades that promote cell growth while inhibiting the commonly with chronic myelogenous (>95%) and less pathways of apoptotic cells. Rin1 is a multifunctional protein that commonly with acute lymphocytic leukemia (<40%). Much has been shown to regulate EGF receptor signaling and research has been focused on understanding the consequence endocytosis. To examine the role of Rin1 in IL3 receptor signaling and cause of this alteration and it has been shown that the pathways, Rin1 and deletion mutants were expressed in cells using Philadelphia chromosome results in the fusion of sequences a retrovirus system. In this study, the overexpression of Rin1 from the BCR and ABL genes (11-18). molecules was shown to selectively block IL-3 activation of the The BCR/ABL fusion product is an activated tyrosine Ras-Erk1/2 and PI3K/Akt pathways and the IL-3-stimulated kinase that confers survival and proliferation advantages to incorporation of [3H] thymidine into DNA without a significant hematopoietic cells, thus contributing to leukemogenesis. effect on the activity of the JNK and p38K pathways. Moreover, Several common points of convergence between cytokine- the depletion of Rin1 by RNA interference induced cell growth. In growth factor signaling and BCR/ABL oncogenesis have been addition, Rin1 was also required as a downstream effector of described and cells expressing BCR/ABL no longer require BCR/ABL-induced cell proliferation. -

Brain Region-Dependent Gene Networks Associated with Selective Breeding for Increased Voluntary Wheel-Running Behavior
UC Riverside UC Riverside Previously Published Works Title Brain region-dependent gene networks associated with selective breeding for increased voluntary wheel-running behavior. Permalink https://escholarship.org/uc/item/8c49g8fd Journal PloS one, 13(8) ISSN 1932-6203 Authors Zhang, Pan Rhodes, Justin S Garland, Theodore et al. Publication Date 2018 DOI 10.1371/journal.pone.0201773 Peer reviewed eScholarship.org Powered by the California Digital Library University of California RESEARCH ARTICLE Brain region-dependent gene networks associated with selective breeding for increased voluntary wheel-running behavior Pan Zhang1,2, Justin S. Rhodes3,4, Theodore Garland, Jr.5, Sam D. Perez3, Bruce R. Southey2, Sandra L. Rodriguez-Zas2,6,7* 1 Illinois Informatics Institute, University of Illinois at Urbana-Champaign, Urbana, IL, United States of America, 2 Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, United a1111111111 States of America, 3 Beckman Institute for Advanced Science and Technology, Urbana, IL, United States of a1111111111 America, 4 Center for Nutrition, Learning and Memory, University of Illinois at Urbana-Champaign, Urbana, a1111111111 IL, United States of America, 5 Department of Evolution, Ecology, and Organismal Biology, University of a1111111111 California, Riverside, CA, United States of America, 6 Department of Statistics, University of Illinois at Urbana-Champaign, Urbana, IL, United States of America, 7 Carle Woese Institute for Genomic Biology, a1111111111 University of Illinois at Urbana-Champaign, Urbana, IL, United States of America * [email protected] OPEN ACCESS Abstract Citation: Zhang P, Rhodes JS, Garland T, Jr., Perez SD, Southey BR, Rodriguez-Zas SL (2018) Brain Mouse lines selectively bred for high voluntary wheel-running behavior are helpful models region-dependent gene networks associated with for uncovering gene networks associated with increased motivation for physical activity and selective breeding for increased voluntary wheel- other reward-dependent behaviors. -

Exploring the Relationship Between Gut Microbiota and Major Depressive Disorders
E3S Web of Conferences 271, 03055 (2021) https://doi.org/10.1051/e3sconf/202127103055 ICEPE 2021 Exploring the Relationship between Gut Microbiota and Major Depressive Disorders Catherine Tian1 1Shanghai American School, Shanghai, China Abstract. Major Depressive Disorder (MDD) is a psychiatric disorder accompanied with a high rate of suicide, morbidity and mortality. With the symptom of an increasing or decreasing appetite, there is a possibility that MDD may have certain connections with gut microbiota, the colonies of microbes which reside in the human digestive system. In recent years, more and more studies started to demonstrate the links between MDD and gut microbiota from animal disease models and human metabolism studies. However, this relationship is still largely understudied, but it is very innovative since functional dissection of this relationship would furnish a new train of thought for more effective treatment of MDD. In this study, by using multiple genetic analytic tools including Allen Brain Atlas, genetic function analytical tools, and MicrobiomeAnalyst, I explored the genes that shows both expression in the brain and the digestive system to affirm that there is a connection between gut microbiota and the MDD. My approach finally identified 7 MDD genes likely to be associated with gut microbiota, implicating 3 molecular pathways: (1) Wnt Signaling, (2) citric acid cycle in the aerobic respiration, and (3) extracellular exosome signaling. These findings may shed light on new directions to understand the mechanism of MDD, potentially facilitating the development of probiotics for better psychiatric disorder treatment. 1 Introduction 1.1 Major Depressive Disorder Major Depressive Disorder (MDD) is a mood disorder that will affect the mood, behavior and other physical parts. -

Altered Gene Expression in Circulating Immune Cells Following a 24-Hour Passive Dehydration
University of Connecticut OpenCommons@UConn Master's Theses University of Connecticut Graduate School 5-10-2020 Altered Gene Expression in Circulating Immune Cells Following a 24-Hour Passive Dehydration Aidan Fiol [email protected] Follow this and additional works at: https://opencommons.uconn.edu/gs_theses Recommended Citation Fiol, Aidan, "Altered Gene Expression in Circulating Immune Cells Following a 24-Hour Passive Dehydration" (2020). Master's Theses. 1480. https://opencommons.uconn.edu/gs_theses/1480 This work is brought to you for free and open access by the University of Connecticut Graduate School at OpenCommons@UConn. It has been accepted for inclusion in Master's Theses by an authorized administrator of OpenCommons@UConn. For more information, please contact [email protected]. Altered Gene Expression in Circulating Immune Cells Following a 24- Hour Passive Dehydration Aidan Fiol B.S., University of Connecticut, 2018 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science At the University of Connecticut 2020 i copyright by Aidan Fiol 2020 ii APPROVAL PAGE Master of Science Thesis Altered Gene Expression in Circulating Immune Cells Following a 24- Hour Passive Dehydration Presented by Aidan Fiol, B.S. Major Advisor__________________________________________________________________ Elaine Choung-Hee Lee, Ph.D. Associate Advisor_______________________________________________________________ Douglas J. Casa, Ph.D. Associate Advisor_______________________________________________________________ Robert A. Huggins, Ph.D. University of Connecticut 2020 iii ACKNOWLEDGEMENTS I’d like to thank my committee members. Dr. Lee, you have been an amazing advisor, mentor and friend to me these past few years. Your advice, whether it was how to be a better writer or scientist, or just general life advice given on our way to get coffee, has helped me grow as a researcher and as a person. -

UC San Diego Electronic Theses and Dissertations
UC San Diego UC San Diego Electronic Theses and Dissertations Title Cardiac Stretch-Induced Transcriptomic Changes are Axis-Dependent Permalink https://escholarship.org/uc/item/7m04f0b0 Author Buchholz, Kyle Stephen Publication Date 2016 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, SAN DIEGO Cardiac Stretch-Induced Transcriptomic Changes are Axis-Dependent A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Bioengineering by Kyle Stephen Buchholz Committee in Charge: Professor Jeffrey Omens, Chair Professor Andrew McCulloch, Co-Chair Professor Ju Chen Professor Karen Christman Professor Robert Ross Professor Alexander Zambon 2016 Copyright Kyle Stephen Buchholz, 2016 All rights reserved Signature Page The Dissertation of Kyle Stephen Buchholz is approved and it is acceptable in quality and form for publication on microfilm and electronically: Co-Chair Chair University of California, San Diego 2016 iii Dedication To my beautiful wife, Rhia. iv Table of Contents Signature Page ................................................................................................................... iii Dedication .......................................................................................................................... iv Table of Contents ................................................................................................................ v List of Figures ................................................................................................................... -

Phenotype Informatics
Freie Universit¨atBerlin Department of Mathematics and Computer Science Phenotype informatics: Network approaches towards understanding the diseasome Sebastian Kohler¨ Submitted on: 12th September 2012 Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) am Fachbereich Mathematik und Informatik der Freien Universitat¨ Berlin ii 1. Gutachter Prof. Dr. Martin Vingron 2. Gutachter: Prof. Dr. Peter N. Robinson 3. Gutachter: Christopher J. Mungall, Ph.D. Tag der Disputation: 16.05.2013 Preface This thesis presents research work on novel computational approaches to investigate and characterise the association between genes and pheno- typic abnormalities. It demonstrates methods for organisation, integra- tion, and mining of phenotype data in the field of genetics, with special application to human genetics. Here I will describe the parts of this the- sis that have been published in peer-reviewed journals. Often in modern science different people from different institutions contribute to research projects. The same is true for this thesis, and thus I will itemise who was responsible for specific sub-projects. In chapter 2, a new method for associating genes to phenotypes by means of protein-protein-interaction networks is described. I present a strategy to organise disease data and show how this can be used to link diseases to the corresponding genes. I show that global network distance measure in interaction networks of proteins is well suited for investigat- ing genotype-phenotype associations. This work has been published in 2008 in the American Journal of Human Genetics. My contribution here was to plan the project, implement the software, and finally test and evaluate the method on human genetics data; the implementation part was done in close collaboration with Sebastian Bauer. -

The Role of the Rho GEF Arhgef2 in RAS Tumorigenesis
The Role of the Rho GEF Arhgef2 in RAS Tumorigenesis by Jane Cullis A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Medical Biophysics University of Toronto © by Jane Cullis 2013 The Role of Rho GEF Arhgef2 in RAS Tumorigenesis Jane Cullis Degree of Doctor of Philosophy, 2013 Graduate Department of Medical Biophysics, University of Toronto Abstract Tumorigenesis is driven by the sequential accumulation of genetic lesions within a cell, each which confer the cell with traits that enable its abnormal growth. The result is a mass of dysregulated cells, or tumor, which, upon further mutation, may spread, or metastasize, to other organs of the body. The dissemination of tumor cells makes treatment difficult, and thus confers cancer with its associated lethality. Over the past 30 years, the RAS genes have been critical in teaching us the mechanisms underlying the molecular progression of cancer. RAS is mutated in 33% of all cancers and is often an early event in its stepwise progression1. As a result, the RAS genes are widely accepted as ‘drivers’ or ‘initiators’ of human tumorigenesis. Unfortunately, efforts directed at targeting RAS in the clinic have as of yet been unsuccessful. This has triggered a need to identify genes that are required for RAS tumorigenesis that are therapeutically tractable. My research has focused on deciphering the potential role of the Rho GEF Arhgef2 in RAS- mediated tumorigenesis. I have found that Arhgef2 is a bona fide transcriptional target of RAS and is upregulated in human tumors harboring RAS mutations. -

Characterization of RIN Isoforms and Their Expression in Tomato Fruit Ripening
cells Article Characterization of RIN Isoforms and Their Expression in Tomato Fruit Ripening Maria A. Slugina, Gleb I. Efremov, Anna V. Shchennikova * and Elena Z. Kochieva Institute of Bioengineering, Research Center of Biotechnology, Russian Academy of Sciences, Leninsky Ave. 33, bld. 2, 119071 Moscow, Russia; [email protected] (M.A.S.); [email protected] (G.I.E.); [email protected] (E.Z.K.) * Correspondence: [email protected]; Tel.: +7-499-1356219 Abstract: Ripening of tomato fleshy fruit is coordinated by transcription factor RIN, which triggers ethylene and carotenoid biosynthesis, sugar accumulation, and cell wall modifications. In this study, we identified and characterized complete sequences of the RIN chromosomal locus in two tomato Solanum lycopersicum cultivars, its rin/RIN genotype, and three wild green-fruited species differing in fruit color and composition. The results reveal that S. lycopersicum cultivars and some wild species (S. pennellii, S. habrochaites, and S. huaylasense) had a 30-splicing site enabling the transcription of RIN1i and RIN2i isoforms. The other wild species (S. arcanum, S. chmielewskii, S. neorickii, and S. peruvianum) had a 30-splicing site only for RIN2i, which was consistent with RIN1i and RIN2i expression patterns. The genotype rin/RIN, which had an extended 30-terminal deletion in the rin allele, mainly expressed the chimeric RIN–MC transcript, which was also found in cultivars (RIN/RIN). The RIN1, but not RIN2, protein is able to induce the transcription of the reporter gene in the Y2H system, which positively correlated with the transcription profile of RIN1i and RIN target genes. We suggest that Citation: Slugina, M.A.; Efremov, during fruit ripening, RIN1 activates ripening-related genes, whereas RIN2 and RIN–MC act as G.I.; Shchennikova, A.V.; Kochieva, modulators by competing for RIN-binding sites in gene promoters, which should be confirmed by E.Z. -
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
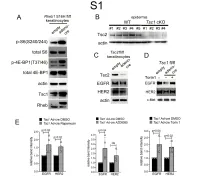
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test).