Nutrition Requirements, Standards, and Operating Bands For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypokalaemia in a Woman with Eating Disorder
Grand Rounds Vol 11 pages 53–55 Specialities: Acute Medicine; Nephrology; Psychiatry Article Type: Case Report DOI: 10.1102/1470-5206.2011.0013 ß 2011 e-MED Ltd Hypokalaemia in a woman with eating disorder Zachary Z. Brenera, Boris Medvedovskya, James F. Winchestera and Michael Bergmanb aDivision of Nephrology, Department of Medicine, Beth Israel Medical Center, Albert Einstein School of Medicine of Yeshiva University, New York, USA; bDepartment of Medicine, Campus Golda, Rabin Medical Center, Petah-Tikva, Tel-Aviv University, Israel Corresponding address: Dr Zachary Z. Brener, 350 E. 17th St., Division of Nephrology, Beth Israel Medical Center, New York, NY 10003, USA. Email: [email protected] Date accepted for publication 13 April 2011 Abstract Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. A concealed diuretic abuse should be suspected, especially in young women with eating disorders. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to early and accurate diagnosis. Keywords Hypokalaemia; eating disorders; diuretics. Introduction Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. After the exclusion of the most obvious causes, a concealed diuretic abuse associated with or without surreptitious vomiting and laxative abuse should be suspected, especially in young women concerned with their body image. A conclusive diagnosis may be difficult as such patients often vigorously deny diuretic intake[1]. Also, only a minority of patients with eating disorders (approximately 6%) abuse diuretics[2–4]. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to an early and accurate diagnosis. -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -
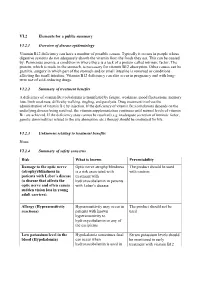
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Essential Trace Elements in Human Health: a Physician's View
Margarita G. Skalnaya, Anatoly V. Skalny ESSENTIAL TRACE ELEMENTS IN HUMAN HEALTH: A PHYSICIAN'S VIEW Reviewers: Philippe Collery, M.D., Ph.D. Ivan V. Radysh, M.D., Ph.D., D.Sc. Tomsk Publishing House of Tomsk State University 2018 2 Essential trace elements in human health UDK 612:577.1 LBC 52.57 S66 Skalnaya Margarita G., Skalny Anatoly V. S66 Essential trace elements in human health: a physician's view. – Tomsk : Publishing House of Tomsk State University, 2018. – 224 p. ISBN 978-5-94621-683-8 Disturbances in trace element homeostasis may result in the development of pathologic states and diseases. The most characteristic patterns of a modern human being are deficiency of essential and excess of toxic trace elements. Such a deficiency frequently occurs due to insufficient trace element content in diets or increased requirements of an organism. All these changes of trace element homeostasis form an individual trace element portrait of a person. Consequently, impaired balance of every trace element should be analyzed in the view of other patterns of trace element portrait. Only personalized approach to diagnosis can meet these requirements and result in successful treatment. Effective management and timely diagnosis of trace element deficiency and toxicity may occur only in the case of adequate assessment of trace element status of every individual based on recent data on trace element metabolism. Therefore, the most recent basic data on participation of essential trace elements in physiological processes, metabolism, routes and volumes of entering to the body, relation to various diseases, medical applications with a special focus on iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), selenium (Se), iodine (I), cobalt (Co), chromium, and molybdenum (Mo) are reviewed. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

Chromium, Vanadium and Ascorbate Effects on Lipids, Cortisol
CHROMIUM, VANADIUM AND ASCORBATE EFFECTS ON LIPIDS, CORTISOL, GLUCOSE AND TISSUE ASCORBATE OF GUINEA PIGS by WOLE KOLA OLADUT, B.S., M.S. A DISSERTATION IN HOME ECONOMICS Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Approved áxigust. tq^ 11;^ ACKNOWLEDGMENTS - <"-^ f/K^ 1 would like to express my sincere gratitude and respect to Dr. Barbara J. Stoecker for her patience, encouragment and direction throughout my graduate program and research project. I am also grateful to other members of my committee, Professors Elizabeth Fox, Donald Oberleas, Marvin Shetlar and Shiang P. Yang. My appreciation is extended to my colleagues, Dr. Reza Zolfaghari for his technical advice, Ms. Gay Riggan, my typist and my coworkers at Methodist Hospital Laboratory. Special thanks are extended to my mother, Mrs. Comfort Oladutemu, my brother, Jide Oladutemu, Dr. Bayode Lasekan and all other family members and friends for their love, help and encouragement during the completion of my graduate education. 11 CONTENTS Page ACKNOWLEDGMENTS il LIST OF TABLES v LIST OF FIGURES viii LIST OF ABBREVIATIONS Ix I. INTRODUCTION 1 II. REVIEW OF LITERATURE 4 Ascorbate and Carbohydrate Metabolism 4 Ascorbate and Lipid Metabolism 6 Chromium and Carbohydrate Metabolism 12 Chromium and Lipid Metabolism 15 Vanadium and Carbohydrate Metabolism 19 Vanadium and Lipid Metabolism 20 Cholesterol-fed Guinea Pigs 22 Conclusion 27 III. ADRENAL ASCORBATE AND CORTISOL CONCENTRATIONS OF GUINEA PIGS SUPPLEMENTED WITH CHROMIUM AND/OR VANADIUM .... 29 Abstract 29 Introduction 30 Materials and Methods 32 Results and Discussion 36 IV. -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Vitamins and Minerals for the Gastroenterologist
VitaminsVitamins andand MineralsMinerals forfor thethe GastroenterologistGastroenterologist AmyAmy Tiu,Tiu, MDMD Feb.Feb. 9,9, 20062006 7:00AM7:00AM conferenceconference ObjectivesObjectives DescriptionDescription fatfat--solublesoluble andand waterwater solublesoluble vitaminsvitamins TraceTrace mineralsminerals (zinc,(zinc, selenium,selenium, iodide,iodide, copper,copper, chromium)chromium) DeficiencyDeficiency andand ToxicityToxicity SourcesSources andand RecommendationsRecommendations ClinicalClinical implicationimplication HistoryHistory 18351835 BritishBritish ParliamentParliament passedpassed thethe MerchantMerchant SeamanSeaman’’ss ActAct thatthat requiredrequired lemonlemon juicejuice toto bebe includedincluded inin thethe rationsrations ofof sailorssailors toto preventprevent scurvyscurvy 19121912 CasimirCasimir FunkFunk coinedcoined thethe termterm vitaminevitamine DailyDaily ValuesValues (DV(DV waswas RDA)RDA) establishedestablished byby thethe NationalNational AcademyAcademy ofof SciencesSciences andand NationalNational ResearchResearch CouncilCouncil asas thethe amountamount toto preventprevent grossgross deficiencydeficiency syndromessyndromes WhichWhich foodfood hashas thethe mostmost vitaminvitamin A?A? Sweet potatoes Beef liver Cantoloupe 1 RE = 10 IU MVI = 3500 IU TPN = 3300 IU VitaminVitamin AA Prevents xerophthalmia (abnormalities in corneal and conjunctival development) Phototransduction Cellular differentiation and integrity of the eye Ancient Egyptians used liver to treat night blindness VitaminVitamin AA -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -

Vitamin and Minerals and Neurologic Disease
Vitamin and Minerals and Neurologic Disease Steven L. Lewis, MD World Congress of Neurology October 2019 Dubai, UAE [email protected] Disclosures . Dr. Lewis has received personal compensation from the American Academy of Neurology for serving as Editor-in-Chief of Continuum: Lifelong Learning in Neurology and for activities related to his role as a director of the American Board of Psychiatry and Neurology, and has received royalty payments from the publishers Wolters Kluwer and Wiley-Blackwell for book authorship. He has no disclosures related to the content or topic of this talk. Objective . Discuss the association of trace mineral deficiencies and vitamin deficiencies (and excess) with neuropathy and myeloneuropathy and other peripheral neurologic syndromes Outline of Presentation . List minerals relevant to neuropathy or myeloneuropathy . Proceed through each mineral and its associated clinical syndrome . List vitamins relevant to neuropathy or myeloneuropathy . Proceed through each vitamin and its associated clinical syndrome Minerals . Naturally occurring nonorganic homogeneous substances . Elements . Required for optimal metabolic and structural processes . Both cations and anions . Essential trace minerals: must be supplied in the diet . Some have recommended daily allowances (RDA) Macrominerals . Sodium . Potassium . Calcium . Magnesium . Phosphorus . Sulfur Macrominerals . Sodium . Potassium . Calcium . Magnesium . Phosphorus . Sulfur Trace Minerals . Chromium . Cobalt . Copper . Iodine . Iron . Manganese . Molybdenum . Selenium . Zinc Trace Minerals . Chromium . Cobalt . Copper . Iodine . Iron . Manganese . Molybdenum . Selenium . Zinc Generalized dose-reponse curve for an essential nutrient Howd and Fan, 2007 Copper . Essential trace element . Human body contains approximately 100 mg Cu . Cofactor of many redox enzymes . Ceruloplasmin most abundant of the cuproenzymes . Involved in antioxidant defense, neuropeptide and blood cell synthesis, and immune function1 1 Bost, J Trace Elements 2016 Copper Deficiency . -

The Serum Sodium, Potassium and Calcium Levels in Children 6- 59 Months of Age with Severe Acute Malnutrition
ORIGINAL ARTICLE The Serum Sodium, Potassium and Calcium Levels in Children 6- 59 Months of Age with Severe Acute Malnutrition BILQUEES FATIMA1, MUHAMMAD AMIN SHEIKH2, MALIK MUHAMMAD NAEEM3 ABSTRACT Aim: To study the serum sodium, potassium and calcium levels in cases of SAM and their comparison in cases with and without diarrhea Methods: This cross sectional study was conducted in the pediatric unit II, Bahawal Victoria Hospital, Bahawalpur from January 2016 to June 2016 in children having age between 6 to 59 months admitting with a diagnosis of SAM. The patients were divided into two groups, Group A with diarrhea and group B without diarrhea. Blood was taken for serum sodium, potassium and calcium. Results: 100 patients were included. Their mean±SD age in months was 23.56±3.80. 62% were males. Serum sodium (mean±SD) was 138.46±4.14 mmol/L, potassium 3.961±0.691 mmol/L and calcium 8.359±0.61 mg/dl. 67 cases presented with (Group A) while 33 without diarrhea (Group B). The serum sodium (mean±SD) was 138.51±4.22 mmol/L in group A while 138.36± 4.05 in group B (p 0.1), the potassium 3.89±0.67 in group A while 4.1±0.72 in group B (p 0.009) and calcium (mg/dL) 8.39±0.6 in group A while 8.34 ±0.73 mg/dL in group B (0.71). There was isolated hyponatremia in 10.4% cases in group A and 9.1% in group B (p 0.83) while isolated hypokalemia in 26.9% cases in group A and 18.2% in group B (p 0.34). -

Pseudo Bartter Syndrome from Surreptitious Purging Behaviour In
al Dis ion ord rit e t rs u N & f T o h Gentile, J Nutr Disorders Ther 2012, 2:1 l e a r n a r DOI: 10.4172/2161-0509.1000107 p u y o Journal of Nutritional Disorders & Therapy J ISSN: 2161-0509 Case Report Open Access Pseudo Bartter Syndrome from Surreptitious Purging Behaviour in Anorexia Nervosa Maria Gabriella Gentile* Eating Disorder Unit, Niguarda Hospital, Piazza Ospedale Maggiore 3, 20126 Milan, Italy Abstract Pseudo Bartter syndrome is a rare disorder characterized by metabolic alkalosis, hypokalaemia, hyperaldosteronism, hyperreninism, normal blood pressure and hyperplasia of the juxtaglomerular apparatus. The most dangerous complication of Pseudo Bartter syndrome is hypokalemia. Hypokalemia caused by vomiting, diarrhea, prolonged fasting, abuse of potassium-depleting drugs, may be present in patients with binge /purging form of anorexia or bulimia nervosa. We report a case of a 19-year-old girl with anorexia nervosa (BMI 16.15 kg/m2) and severe prolonged hypokalemia (1.9 mEq/l), metabolic alkalosis and severe protracted secondary hyperaldosteronism (i.e. Pseudo Bartter’s syndrome) from surreptitious purging behaviour (vomit and laxative abuse). An intensive multidisciplinary day-hospital treatment including long-term potassium supplementation, a potassium- sparing diuretic was necessary to resolve the case and to allow the young girl to admit her previous purging behaviour and after three months to get at a normal kalemia without any potassium supplementation and BMI at a normal value (20 kg/m2). Given the dangers to the heart electrical and mechanical functions set by severe potassium deficiency, it is mandatory to find out the true cause so that a proper treatment can be started.