Cell Potentials a Galvanic Cell Is a Combination of an Anode And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrochemical Cells
Electrochemical cells = electronic conductor If two different + surrounding electrolytes are used: electrolyte electrode compartment Galvanic cell: electrochemical cell in which electricity is produced as a result of a spontaneous reaction (e.g., batteries, fuel cells, electric fish!) Electrolytic cell: electrochemical cell in which a non-spontaneous reaction is driven by an external source of current Nils Walter: Chem 260 Reactions at electrodes: Half-reactions Redox reactions: Reactions in which electrons are transferred from one species to another +II -II 00+IV -II → E.g., CuS(s) + O2(g) Cu(s) + SO2(g) reduced oxidized Any redox reactions can be expressed as the difference between two reduction half-reactions in which e- are taken up Reduction of Cu2+: Cu2+(aq) + 2e- → Cu(s) Reduction of Zn2+: Zn2+(aq) + 2e- → Zn(s) Difference: Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq) - + - → 2+ More complex: MnO4 (aq) + 8H + 5e Mn (aq) + 4H2O(l) Half-reactions are only a formal way of writing a redox reaction Nils Walter: Chem 260 Carrying the concept further Reduction of Cu2+: Cu2+(aq) + 2e- → Cu(s) In general: redox couple Ox/Red, half-reaction Ox + νe- → Red Any reaction can be expressed in redox half-reactions: + - → 2 H (aq) + 2e H2(g, pf) + - → 2 H (aq) + 2e H2(g, pi) → Expansion of gas: H2(g, pi) H2(g, pf) AgCl(s) + e- → Ag(s) + Cl-(aq) Ag+(aq) + e- → Ag(s) Dissolution of a sparingly soluble salt: AgCl(s) → Ag+(aq) + Cl-(aq) − 1 1 Reaction quotients: Q = a − ≈ [Cl ] Q = ≈ Cl + a + [Ag ] Ag Nils Walter: Chem 260 Reactions at electrodes Galvanic cell: -

On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 Against Metal Electrodes
nanomaterials Article Fluoride-Ion Batteries: On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 against Metal Electrodes Maria Gombotz 1,* , Veronika Pregartner 1, Ilie Hanzu 1,2,* and H. Martin R. Wilkening 1,2 1 Institute for Chemistry and Technology of Materials, Technical Universtiy of Graz, Graz 8010, Austria; [email protected] (V.P.); [email protected] (H.M.R.W.) 2 ALISTORE—European Research Institute, CNRS FR3104, Hub de l’Energie, Rue Baudelocque, 80039 Amiens, France * Correspondence: [email protected] (M.G.); [email protected] (I.H.) Received: 27 September 2019; Accepted: 23 October 2019; Published: 25 October 2019 Abstract: Over the past years, ceramic fluorine ion conductors with high ionic conductivity have stepped into the limelight of materials research, as they may act as solid-state electrolytes in fluorine-ion batteries (FIBs). A factor of utmost importance, which has been left aside so far, is the electrochemical stability of these conductors with respect to both the voltage window and the active materials used. The compatibility with different current collector materials is important as well. In the course of this study, tysonite-type La0.9Ba0.1F2.9, which is one of the most important electrolyte in first-generation FIBs, was chosen as model substance to study its electrochemical stability against a series of metal electrodes viz. Pt, Au, Ni, Cu and Ag. To test anodic or cathodic degradation processes we carried out cyclic voltammetry (CV) measurements using a two-electrode set-up. We covered a voltage window ranging from −1 to 4 V, which is typical for FIBs, and investigated the change of the response of the CVs as a function of scan rate (2 mV/s to 0.1 V/s). -

Galvanic Cell Notation • Half-Cell Notation • Types of Electrodes • Cell
Galvanic Cell Notation ¾Inactive (inert) electrodes – not involved in the electrode half-reaction (inert solid conductors; • Half-cell notation serve as a contact between the – Different phases are separated by vertical lines solution and the external el. circuit) 3+ 2+ – Species in the same phase are separated by Example: Pt electrode in Fe /Fe soln. commas Fe3+ + e- → Fe2+ (as reduction) • Types of electrodes Notation: Fe3+, Fe2+Pt(s) ¾Active electrodes – involved in the electrode ¾Electrodes involving metals and their half-reaction (most metal electrodes) slightly soluble salts Example: Zn2+/Zn metal electrode Example: Ag/AgCl electrode Zn(s) → Zn2+ + 2e- (as oxidation) AgCl(s) + e- → Ag(s) + Cl- (as reduction) Notation: Zn(s)Zn2+ Notation: Cl-AgCl(s)Ag(s) ¾Electrodes involving gases – a gas is bubbled Example: A combination of the Zn(s)Zn2+ and over an inert electrode Fe3+, Fe2+Pt(s) half-cells leads to: Example: H2 gas over Pt electrode + - H2(g) → 2H + 2e (as oxidation) + Notation: Pt(s)H2(g)H • Cell notation – The anode half-cell is written on the left of the cathode half-cell Zn(s) → Zn2+ + 2e- (anode, oxidation) + – The electrodes appear on the far left (anode) and Fe3+ + e- → Fe2+ (×2) (cathode, reduction) far right (cathode) of the notation Zn(s) + 2Fe3+ → Zn2+ + 2Fe2+ – Salt bridges are represented by double vertical lines ⇒ Zn(s)Zn2+ || Fe3+, Fe2+Pt(s) 1 + Example: A combination of the Pt(s)H2(g)H Example: Write the cell reaction and the cell and Cl-AgCl(s)Ag(s) half-cells leads to: notation for a cell consisting of a graphite cathode - 2+ Note: The immersed in an acidic solution of MnO4 and Mn 4+ reactants in the and a graphite anode immersed in a solution of Sn 2+ overall reaction are and Sn . -

Electrochemical Cells - Redox Reactions Can Be Used in a Controlled Manner to Make a Battery
Chapter 17 Worksheet #2 Name __________________________ Electrochemical Cells - Redox reactions can be used in a controlled manner to make a battery. A galvanic cell (voltaic cell or battery) converts the chemical energy of the reactants into electrical energy. BATTERY: Anode - AN OX, RED CAT Cathode - Salt Bridge - A tube containing a salt (such as KCl or NaNO3) solution that is used to connect two half-cells in an electrochemical cell; allows the passage of ions (maintains charge neutrality), but prevents the mixing of half-cell electrolytes. Shorthand notation for a galvanic cell: Zn(s)│Zn2+(aq)║Cu2+(aq)│Cu(s) where the anode is on the left, the cathode on the right, │ indicates the interface between the metal and solution, and ║ indicates the salt bridge. In many cells, the electrode itself does not react but serves only as a channel to direct electrons to or from the solution where a reaction involving other species takes place. The electrode itself is unaffected. Platinum and graphite are inert in most (but not all) electrochemical reactions. The Cu electrode could be replaced by a platinum or graphite electrode in the Zn/Cu battery: Zn(s)│Zn2+(aq)║Cu2+(aq)│Pt(s) Construct a battery from the reaction: Cr(s) + Pb2+(aq) Cr3+(aq) + Pb(s) Construct a galvanic cell using platinum electrodes and the reaction: - - + 2+ 10 Br (aq) + 2 MnO4 (aq) + 16 H (aq) 5 Br2(ℓ) + 2 Mn (aq) + 8 H2O(ℓ) A salt bridge is not required in a battery in which the reactants are physically separated from each other. -

Electrochemistry: Elektrolytic and Galvanic Cell Co08 Galvanic Series (Beketov, Cca 1860)
1/26 Electrochemistry: Elektrolytic and galvanic cell co08 Galvanic series (Beketov, cca 1860): Li, Ca, Al, Mn, Cr Zn, Cd Fe, Pb, [H2], Cu, Ag, Au ≈ ≈ ⊕ Cell = system composed of two electrodes and an electrolyte. electrolytic cell: electric energy chemical reaction ! galvanic cell: chemical reaction electric energy ! reversible galvanic cell (zero current) Electrodes anode = electrode where oxidation occurs Cu Cu2+ + 2 e ! − 2 Cl Cl2 + 2 e − ! − cathode = electrode where reduction occurs 2 Cu + + 2 e Cu credit: Wikipedia (free) − ! Cl2 + 2 e 2 Cl − ! − Oxidation and reduction are separated in a cell. The charge flows through the circuit. 2/26 Anode and cathode co08 electrolytic cell galvanic cell '$ '$ - - ⊕&% &% ⊕ Cl2 Cu2+ Cu2+ Pt Cl ! ! 2 Cu Cl Cl − Cu − CuCl2(aq) CuCl2(aq) anode cathode anode cathode “anions go to the anode” 3/26 Galvanic cells: electrodes, convention co08 Electrodes(= half-cells) may be separated by a porous separator, polymeric mem- brane, salt bridge. Cathode is right (reduction) ⊕ Anode is left (oxidation) negative electrode (anode) positive electrode (cathode) ⊕ liquid junction phase boundary . (porous separator) j salt bridge .. semipermeable membrane k Examples: 3 Cu(s) CuCl2(c = 0.1 mol dm ) Cl2(p = 95 kPa) Pt j − j j ⊕ Ag s AgCl s NaCl m 4 mol kg 1 Na(Hg) ( ) ( ) ( = − ) j j j 1 NaCl(m = 0.1 mol kg ) AgCl(s) Ag(s) j − j j ⊕ 2 3 4 3 3 3 Pt Sn +(0.1 mol dm ) + Sn +(0.01 mol dm ) Fe +(0.2 mol dm ) Fe j − − jj − j ⊕ 4/26 Equilibrium cell potential co08 Also: electromotive potential/voltage, electromo- tive force (EMF). -

Thin Films of Lithium Ion Conducting Garnets and Their Properties
Thin films of lithium ion conducting garnets and their properties dem Fachbereich Biologie und Chemie der Justus-Liebig-Universität Gießen vorgelegte Dissertation zur Erlangung des Grades Doktor der Naturwissenschaften – Dr. rer. nat. – von Jochen Reinacher geboren am 07.09.1984 Gießen 2014 II Dekan / Dean Prof. Dr. Holger Zorn 1. Gutachter / Reviewer Prof. Dr. Jürgen Janek 2. Gutachter / Reviewer Prof. Dr. Bruno K. Meyer Arbeit eingereicht: 10.06.2014 Tag der mündlichen Prüfung: 11.07.2014 III IV Abstract Different lithium ion conducting garnet-type thin films were prepared by pulsed laser deposition. Of these garnet-type thin films Li6BaLa2Ta2O12, cubic Li6.5La3Zr1.5Ta0.5O12 (additionally stabilized by Al2O3) and cubic Li7La3Zr2O12 stabilized by Ga2O3 were investigated in more detail. Conductivity measurements of these thin films performed in lateral geometry showed total conductivities of σ = 1.7∙10–6 S∙cm–1, σ = 2.9∙10–6 S∙cm–1 and σ = 1.2∙10–6 S∙cm–1, respectively. Electrochemical impedance spectroscopy was performed in axial geometry (orthogonal to the substrate), revealing conductivities of –5 –1 σ = 3.3∙10 S∙cm for Li6BaLa2Ta2O12 which is comparable to the bulk conductivity of –5 –1 Li6BaLa2Ta2O12 (σ = 4∙10 S∙cm ). The comparatively low lateral conductivity of the garnet-type material could be increased to a maximum of σ = 2.8∙10–5 S∙cm–1 for multilayer structures of two different alternating garnet-type materials (Li6.5La3Zr1.5Ta0.5O12:Al2O3, Li7La3Zr2O12:Ga2O3). These investigations revealed a strong influence of the thin film microstructure on the total conductivity. Additionally the electronic partial conductivity of Li6BaLa2Ta2O12 as bulk and thin film material was determined. -

Electrochemistry –An Oxidizing Agent Is a Species That Oxidizes Another Species; It Is Itself Reduced
Oxidation-Reduction Reactions Chapter 17 • Describing Oxidation-Reduction Reactions Electrochemistry –An oxidizing agent is a species that oxidizes another species; it is itself reduced. –A reducing agent is a species that reduces another species; it is itself oxidized. Loss of 2 e-1 oxidation reducing agent +2 +2 Fe( s) + Cu (aq) → Fe (aq) + Cu( s) oxidizing agent Gain of 2 e-1 reduction Skeleton Oxidation-Reduction Equations Electrochemistry ! Identify what species is being oxidized (this will be the “reducing agent”) ! Identify what species is being •The study of the interchange of reduced (this will be the “oxidizing agent”) chemical and electrical energy. ! What species result from the oxidation and reduction? ! Does the reaction occur in acidic or basic solution? 2+ - 3+ 2+ Fe (aq) + MnO4 (aq) 6 Fe (aq) + Mn (aq) Steps in Balancing Oxidation-Reduction Review of Terms Equations in Acidic solutions 1. Assign oxidation numbers to • oxidation-reduction (redox) each atom so that you know reaction: involves a transfer of what is oxidized and what is electrons from the reducing agent to reduced 2. Split the skeleton equation into the oxidizing agent. two half-reactions-one for the oxidation reaction (element • oxidation: loss of electrons increases in oxidation number) and one for the reduction (element decreases in oxidation • reduction: gain of electrons number) 2+ 3+ - 2+ Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) 1 3. Complete and balance each half reaction Galvanic Cell a. Balance all atoms except O and H 2+ 3+ - 2+ (Voltaic Cell) Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) b. -

Battery Technologies for Small Scale Embeded Generation
Battery Technologies for Small Scale Embedded Generation. by Norman Jackson, South African Energy Storage Association (SAESA) Content Provider – Wikipedia et al Small Scale Embedded Generation - SSEG • SSEG is very much a local South African term for Distributed Generation under 10 Mega Watt. Internationally they refer to: Distributed generation, also distributed energy, on-site generation (OSG) or district/decentralized energy It is electrical generation and storage performed by a variety of small, grid- connected devices referred to as distributed energy resources (DER) Types of Energy storage: • Fossil fuel storage • Thermal • Electrochemical • Mechanical • Brick storage heater • Compressed air energy storage • Cryogenic energy storage (Battery Energy • Fireless locomotive • Liquid nitrogen engine Storage System, • Flywheel energy storage • Eutectic system BESS) • Gravitational potential energy • Ice storage air conditioning • Hydraulic accumulator • Molten salt storage • Flow battery • Pumped-storage • Phase-change material • Rechargeable hydroelectricity • Seasonal thermal energy battery • Electrical, electromagnetic storage • Capacitor • Solar pond • UltraBattery • Supercapacitor • Steam accumulator • Superconducting magnetic • Thermal energy energy storage (SMES, also storage (general) superconducting storage coil) • Chemical • Biological • Biofuels • Glycogen • Hydrated salts • Starch • Hydrogen storage • Hydrogen peroxide • Power to gas • Vanadium pentoxide History of the battery This was a stack of copper and zinc Italian plates, -

Galvanic Cells and the Nernst Equation
Exercise 7 Page 1 Illinois Central College CHEMISTRY 132 Name:___________________________ Laboratory Section: _______ Galvanic Cells and the Nernst Equation Equipment Voltage probe wires 0.1 M solutions of Pb(NO3)2, Fe(NO3)3, and KNO3 sandpaper or steel wool 0.1 M solutions of Cu(NO3)2 and Zn(NO3)2 plastic document protector 1.0 M solutions of Cu(NO3)2 and Zn(NO3)2 6 x 1.5 cm strips of filter paper Cu(NO3)2: 0.010 M, 0.0010 M, and 0.00010 M Objectives. The objectives of this experiment are to develop an understanding of the "Electrochemical Series" and to illustrate the use the Nernst Equation. Background Any chemical reaction involving the transfer of electrons from one substance to another is an oxidation-reduction (redox) reaction. The substance losing electrons is oxidized while the substance gaining electrons is reduced. Let us consider the following redox reaction: +2 +2 Zn(s) + Pb (aq) Zn (aq) + Pb(s) This redox reaction can be divided into an oxidation and a reduction half-reaction. +2 -1 Zn(s) Zn (aq) + 2 e oxidation half-reaction +2 -1 Pb (aq) + 2 e Pb(s) reduction half-reaction A galvanic cell (Figure 1.) is a device used to separate a redox reaction into its two component half-reactions in such a way that the electrons are forced to travel through an external circuit rather than by direct contact of the oxidizing agent and reducing agent. This transfer of electrons through an external circuit is electricity. Figure 1. Exercise 7 Page 2 Each side of the galvanic cell is known as a half-cell. -

Physical Chemistry LD
Physical Chemistry LD Electrochemistry Chemistry Galvanic elements Leaflets C4.4.4.2a Leclanché cell Time required: 120 – 150 min + over night Aims of the experiment To construct a galvanic cell. To examine the Leclanché cell as a galvanic cell. To follow the path from galvanic cell to battery. The zinc-carbon battery and its characteristic curve. Principles casing In 1780, the Italian researcher and physician L. Galvani no- insula- ticed that a frog leg jerks when it comes into contact with iron tor and copper. He presumed that there was an electric effect at work. 20 years later, A. Volta developed the first battery (pri- metal mary cell) in the form of the voltaic pile. Further developments cup in the “wet battery field” followed. However, none of these cells were suitable for everyday use since the electrolyte was zinc in the form of a liquid. They are therefore not useful for mobile use for such things as pocket flash lights, mobile telephones, etc. Finally, in 1866 the first dry battery was patented, at that NH4Cl time still in wet form, by French physical chemist G. Leclan- ché. In the process, the electrolyte solution was turned into a gel using binders so that mobile use was possible. MnO2/C The Leclanché cell has a (clamping) voltage of 1.5 V. The graphite anode is made of zinc and the cathode is made of manga- electrode nese dioxide and carbon. A thickened ammonium chloride solution is used as the electrolyte. Additionally, zinc chloride is used in order to increase the energy density (charging density) (see Fig. -

A Fuel Cell Is an Electrochemical Device (A Galvanic Cell) Which
INTRODUCTION TO FUEL CELLS R.K. Shah Subros Ltd., Noida, UP, India, E-mail: [email protected] A fuel cell is an electrochemical device (a galvanic cell) which converts free energy of a chemical reaction into electrical energy (electricity); byproducts are heat and water/steam if hydrogen and air are the reactants; in some fuel cell types, the additional byproducts may be carbon dioxide and leftover lower forms of hydrocarbons depending on the fossil fuels used. There is no combustion in this process and hence no NOx are generated. Sulfur is poison to all fuel cells so it must be removed from any fuel before feeding to any fuel cell type. A fuel cell produces electricity on demand continuously as long as the fuel and oxidant are supplied. For reference, primary cell or battery is also an electrochemical energy producing device (one-way chemical reaction producing electricity) and needs to throw away once the battery is discharged. A rechargeable or secondary battery is an electrochemical energy storage device having reversible chemical reaction producing or using electricity, but it also has a limited life. The components of a fuel cell are anode, anodic catalyst layer, electrolyte, cathodic catalyst layer cathode, bipolar plates/interconnects and sometimes gaskets for sealing/preventing leakage of gases between anode and cathode. The stack of such fuel cells (a repeated stack of such components) is connected in series/parallel connections to yield the desired voltage and current. The anode and cathode consist of porous gas diffusion layers, usually made of highly electron conductivity materials (and having zero proton conductivity theoretically) such as porous graphite thin layers. -
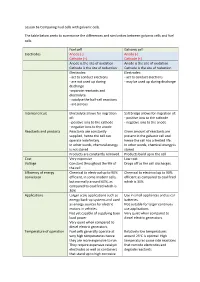
Lesson 6A Comparing Fuel Cells with Galvanic Cells. the Table Below Seeks to Summarise the Differences and Similarities Between
Lesson 6a Comparing Fuel cells with galvanic cells. The table below seeks to summarise the differences and similarities between galvanic cells and fuel cells. Fuel cell Galvanic cell Electrodes Anode (-) Anode (-) Cathode (+) Cathode (+) Anode is the site of oxidation Anode is the site of oxidation Cathode is the site of reduction Cathode is the site of reduction Electrodes: Electrodes: - act to conduct electrons - act to conduct electrons - are not used up during - may be used up during discharge discharge -separate reactants and electrolyte - catalyse the half-cell reactions - are porous Internal circuit Electrolyte allows for migration Salt bridge allows for migration of: of: - positive ions to the cathode - positive ions to the cathode - negative ions to the anode - negative ions to the anode Reactants and products Reactants are constantly Given amount of reactants are supplied, hence the cell can present in the galvanic cell and operate indefinitely. hence the cell has a limited life. In other words, chemical energy In other words, chemical energy is is not stored stored Products are constantly removed. Products build up in the cell Cost Very expensive Low cost Voltage Constant throughout the life of Drops off as the cell discharges. the cell Efficiency of energy Chemical to electrical up to 90% Chemical to electrical up to 90% conversion efficient, in some modern cells, efficient as compared to coal fired but normally around 60%, as which is 30% compared to coal fired which is 30% Applications Larger scale applications such as Use in small appliances and as car energy back-up systems and used batteries.