This Is the Peer Reviewed Version of the Following Article: Borger, C. and Riethmuller, G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Paleobotanical Data on the Portuguese Pennsylvanian (Douro Carboniferous Basin, NW Portugal)
Versão online: http://www.lneg.pt/iedt/unidades/16/paginas/26/30/185 Comunicações Geológicas (2014) 101, Especial I, 409-414 IX CNG/2º CoGePLiP, Porto 2014 ISSN: 0873-948X; e-ISSN: 1647-581X New paleobotanical data on the Portuguese Pennsylvanian (Douro Carboniferous Basin, NW Portugal) Novos dados paleobotânicos do Pensilvaniano português (Bacia Carbonífera do Douro, NW Portugal) P. Correia1*, Z. Šimůnek2, J. Pšenička3, A. A. Sá4,5, R. Domingos6, A. Carneiro7, D. Flores1,7 Artigo Curto Short Article © 2014 LNEG – Laboratório Nacional de Geologia e Energia IP Abstract: This paper describes nine new macrofloral taxa from 1. Introduction Douro Carboniferous Basin (lower Gzhelian) of Portugal. The plant assemblage is mainly composed by pteridophylls (Sphenopteriss The fossil flora of Carboniferous of Portugal is still little arberi Kidston, Sphenopteris fayoli Zeiller, Sphenopteris tenuis known. The new megafloral occurrences recently found in Schenk, Odontopteris schlotheimii Brongniart), sphenopsids the Upper Pennsylvanian strata of Douro Carboniferous (Annularia spicata Gutbier, Stellotheca robusta (Feistmantel) Basin (DCB) provide new and important data about Surange and Prakash, Calamostachys grandis Zeiller (Jongmans) and Calamostachys calathifera Sterzel) besides the gymnosperm paleobotanical richness and diversity of the Paleozoic Cordaites foliolatus Grand`Eury. The new data provide a better floras of Portugal offering more information to previous understating of the knowledge of Late Carboniferous floras of researches reported from diverse localities and by different Portugal, showing the high plant diversity of Gzhelian floras, when authors (e.g. Wenceslau de Lima, Bernardino António considerable changes in paleogeography and climate dynamics are Gomes, Carlos Ribeiro, Carríngton da Costa, Carlos evidenced in Euramerican floristic assemblages. -

Evolution of the Female Conifer Cone Fossils, Morphology and Phylogenetics
DEPARTMENT OF BIOLOGICAL AND ENVIRONMENTAL SCIENCES EVOLUTION OF THE FEMALE CONIFER CONE FOSSILS, MORPHOLOGY AND PHYLOGENETICS Daniel Bäck Degree project for Bachelor of Science with a major in Biology BIO602, Biologi: Examensarbete – kandidatexamen, 15 hp First cycle Semester/year: Spring 2020 Supervisor: Åslög Dahl, Department of Biological and Environmental Sciences Examiner: Claes Persson, Department of Biological and Environmental Sciences Front page: Abies koreana (immature seed cones), Gothenburg Botanical Garden, Sweden Table of contents 1 Abstract ............................................................................................................................... 2 2 Introduction ......................................................................................................................... 3 2.1 Brief history of Florin’s research ............................................................................... 3 2.2 Progress in conifer phylogenetics .............................................................................. 4 3 Aims .................................................................................................................................... 4 4 Materials and Methods ........................................................................................................ 4 4.1 Literature: ................................................................................................................... 4 4.2 RStudio: ..................................................................................................................... -

A Comparative Study of the Primary Vascular System Of
ArneI'. J. Bot. 5.5(4): 447-457. 1968. A COMPARATIVE STUDY OF THE PRIMARY VASCULAR SYSTElVI OF CONIFERS. 1. GENERA WITH HELICAL PHYLLOTAXISl KADAMBARI K. N AMBOODIRI2 AND CHARLES B. BECK Department of Botany, University of Michigan, Ann Arbor ABSTRACT The primary vascular system of 23 species belonging to 18 genera of conifers with helical phyllotaxis has been investigated with the intent of determining the architecture .f the system. Special attention has been given to nodal and subnodal relations of the vascular bundles. The vascular system seems to be composed solely of relatively discrete sympodia, that is, axial vascu lar bundles from which leaf traces branch unilaterally. Although the discreteness of the syrn podia is not immediately apparent because of their undulation and lateral contacts with neigh boring ones, close examination, including a statistical analysis of the tangential contacts, seems to reveal that each sympodium maintains its identity throughout. Although two traces may be apparent at nodal levels, the trace supply to a leaf originates, in all species, as a single bundle. An analysis is made of the relationship between the vasculature and the phyllotaxis. It is ob served that the direction of trace divergence can be accurately predicted when the direction of the ontogenetic spiral, the angle of divergence of leaf traces, and the number of syrnpodia are known. THE ORIGIN and evolution of gymnosperms that of the ferns by reduction (Jeffrey, 1902, are significant problems that deserve increased 1917). Consequently, he considered the leaf gap attention. There have been few modern compara of seed plants to be homologous with that of tive studies of extant gymnosperms, and most the ferns. -

Pollination Drop in Relation to Cone Morphology in Podocarpaceae: a Novel Reproductive Mechanism Author(S): P
Pollination Drop in Relation to Cone Morphology in Podocarpaceae: A Novel Reproductive Mechanism Author(s): P. B. Tomlinson, J. E. Braggins, J. A. Rattenbury Source: American Journal of Botany, Vol. 78, No. 9 (Sep., 1991), pp. 1289-1303 Published by: Botanical Society of America Stable URL: http://www.jstor.org/stable/2444932 . Accessed: 23/08/2011 15:47 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Botanical Society of America is collaborating with JSTOR to digitize, preserve and extend access to American Journal of Botany. http://www.jstor.org AmericanJournal of Botany 78(9): 1289-1303. 1991. POLLINATION DROP IN RELATION TO CONE MORPHOLOGY IN PODOCARPACEAE: A NOVEL REPRODUCTIVE MECHANISM' P. B. TOMLINSON,2'4 J. E. BRAGGINS,3 AND J. A. RATTENBURY3 2HarvardForest, Petersham, Massachusetts 01366; and 3Departmentof Botany, University of Auckland, Auckland, New Zealand Observationof ovulatecones at thetime of pollinationin the southernconiferous family Podocarpaceaedemonstrates a distinctivemethod of pollencapture, involving an extended pollinationdrop. Ovules in all generaof the family are orthotropousand singlewithin the axil of each fertilebract. In Microstrobusand Phyllocladusovules are-erect (i.e., the micropyle directedaway from the cone axis) and are notassociated with an ovule-supportingstructure (epimatium).Pollen in thesetwo genera must land directly on thepollination drop in theway usualfor gymnosperms, as observed in Phyllocladus.In all othergenera, the ovule is inverted (i.e., the micropyleis directedtoward the cone axis) and supportedby a specializedovule- supportingstructure (epimatium). -

X. the Conifers and Ginkgo
X. The Conifers and Ginkgo Now we turn our attention to the Coniferales, another great assemblage of seed plants. First let's compare the conifers with the cycads: Cycads Conifers few apical meristems per plant many apical meristems per plant leaves pinnately divided leaves undivided wood manoxylic wood pycnoxylic seeds borne on megaphylls seeds borne on stems We should also remember that these two groups have a lot in common. To begin with, they are both groups of woody seed plants. They are united by a small set of derived features: 1) the basic structure of the stele (a eustele or a sympodium, two words for the same thing) and no leaf gaps 2) the design of the apical meristem (many initials, subtended by a slowly dividing group of cells called the central mother zone) 3) the design of the tracheids (circular-bordered pits with a torus) We have three new seed plant orders to examine this week: A. Cordaitales This is yet another plant group from the coal forest. (Find it on the Peabody mural!) The best-known genus, Cordaites, is a tree with pycnoxylic wood bearing leaves up to about a foot and a half long and four inches wide. In addition, these trees bore sporangia (micro- and mega-) in strobili in the axils of these big leaves. The megasporangia were enclosed in ovules. Look at fossils of leaves and pollen-bearing shoots of Cordaites. The large, many-veined megaphylls are ancestral to modern pine needles; the shoots are ancestral to pollen-bearing strobili of modern conifers. 67 B. -

Retallack 2021 Coal Balls
Palaeogeography, Palaeoclimatology, Palaeoecology 564 (2021) 110185 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Modern analogs reveal the origin of Carboniferous coal balls Gregory Retallack * Department of Earth Science, University of Oregon, Eugene, Oregon 97403-1272, USA ARTICLE INFO ABSTRACT Keywords: Coal balls are calcareous peats with cellular permineralization invaluable for understanding the anatomy of Coal ball Pennsylvanian and Permian fossil plants. Two distinct kinds of coal balls are here recognized in both Holocene Histosol and Pennsylvanian calcareous Histosols. Respirogenic calcite coal balls have arrays of calcite δ18O and δ13C like Carbon isotopes those of desert soil calcic horizons reflecting isotopic composition of CO2 gas from an aerobic microbiome. Permineralization Methanogenic calcite coal balls in contrast have invariant δ18O for a range of δ13C, and formed with anaerobic microbiomes in soil solutions with bicarbonate formed by methane oxidation and sugar fermentation. Respiro genic coal balls are described from Holocene peats in Eight Mile Creek South Australia, and noted from Carboniferous coals near Penistone, Yorkshire. Methanogenic coal balls are described from Carboniferous coals at Berryville (Illinois) and Steubenville (Ohio), Paleocene lignites of Sutton (Alaska), Eocene lignites of Axel Heiberg Island (Nunavut), Pleistocene peats of Konya (Turkey), and Holocene peats of Gramigne di Bando (Italy). Soils and paleosols with coal balls are neither common nor extinct, but were formed by two distinct soil microbiomes. 1. Introduction and Royer, 2019). Although best known from Euramerican coal mea sures of Pennsylvanian age (Greb et al., 1999; Raymond et al., 2012, Coal balls were best defined by Seward (1895, p. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Taphonomy As an Aid to African Palaeontology*
Palaeont. afr., 24 (1981 ) PRESIDENTIAL ADDRESS: TAPHONOMY AS AN AID TO AFRICAN PALAEONTOLOGY* by C.K. Brain Transvaal Museum, P.O. Box 413, Pretoria 0001 SUMMARY Palaeontology has its roots in both the earth and life sciences. Its usefulness to geology comes from the light which the understanding of fossils may throw on the stratigraphic re lationships of sediments, or the presence of economic deposits such as coal or oil. In biology, the study of fossils has the same objectives as does the study of living animals or plants and such objectives are generally reached in a series of steps which may be set out as follows: STEP I. Discovering what forms of life are, or were, to be found in a particular place at a particular time. Each form is allocated a name and is fitted into a system of classification. These contributions are made by the taxonomist or the systematist. STEP 2. Gaining afuller understanding ofeach described taxon as a living entity. Here the input is from the anatomist, developmental biologist, genetIcIst, physi ologist or ethologist and the information gained is likely to modify earlier decisions taken on the systematic position of the forms involved. STEP 3. Understanding the position ofeach form in the living community or ecosystem. This step is usually taken by a population biologist or ecologist. Hopefully, any competent neo- or palaeobiologist (I use the latter term deliberately in this context in preference to "palaeontologist") should be able to contribute to more than one of the steps outlined above. Although the taxonomic and systematic steps have traditionally been taken in museums or related institutions, it is encouraging to see that some of the steps subsequent to these very basic classificatory ones are now also being taken by museum biol ogists. -
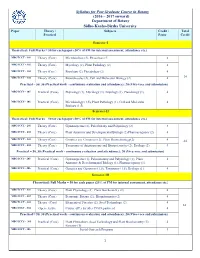
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

International Organisation of Palaeobotany IOP NEWSLETTER
IOP 107 September 2015 INTERNATIONAL UNION OF BIOLOGICAL SCIENCES SECTION FOR PALAEOBOTANY International Organisation of Palaeobotany IOP NEWSLETTER 107 September 2015 CONTENTS FROM THE SECRETARY/TREASURER IPC XIV/IOPC X 2016 MEETING REPORT OBITUARY BOOK REVIEW UPCOMING MEETINGS CALL FOR NEWS and NOTES The views expressed in the newsletter are those of its correspondents, and do not necessarily reflect the policy of IOP. Please send us your contributions for the next edition of our newsletter (December 2015) by November 30th, 2015. President: Johanna Eder-Kovar (Germany) Vice Presidents: Bob Spicer (Great Britain), Harufumi Nishida (Japan), Mihai Popa (Romania) Members at Large: Jun Wang (China), Hans Kerp (Germany), Alexej Herman (Russia) Secretary/Treasurer/Newsletter editor: Mike Dunn (USA) Conference/Congress Chair: Francisco de Assis Ribeiro dos Santos IOP Logo: The evolution of plant architecture (© by A. R. Hemsley) IOP 107 1 September 2015 FROM THE and also, who you want to run your organization. A call for nominees to the SECRETARY/TREASURER Executive Council will go out in the December Newsletter. Dear International Organisation of Palaeobotany Members, Please feel free to contact me with questions, comments, or any information Please accept this July-ish newsletter. you would like passed on to the Membership. I can be reached at: Thanks to everyone who submitted items for the Newsletter. I really appreciate the Mike Dunn support of those who sent items in. The Department of Biological Sciences International Organisation of Palaeobotany Cameron University is a Member-Centric Organization, and this Lawton, Oklahoma 73505 Newsletter is an example of how great and Ph.: 580-581-2287 meaningful we can be when the membership email: [email protected] participates. -

Curriculum Vitae
CURRICULUM VITAE ORCID ID: 0000-0003-0186-6546 Gar W. Rothwell Edwin and Ruth Kennedy Distinguished Professor Emeritus Department of Environmental and Plant Biology Porter Hall 401E T: 740 593 1129 Ohio University F: 740 593 1130 Athens, OH 45701 E: [email protected] also Courtesy Professor Department of Botany and PlantPathology Oregon State University T: 541 737- 5252 Corvallis, OR 97331 E: [email protected] Education Ph.D.,1973 University of Alberta (Botany) M.S., 1969 University of Illinois, Chicago (Biology) B.A., 1966 Central Washington University (Biology) Academic Awards and Honors 2018 International Organisation of Palaeobotany lifetime Honorary Membership 2014 Fellow of the Paleontological Society 2009 Distinguished Fellow of the Botanical Society of America 2004 Ohio University Distinguished Professor 2002 Michael A. Cichan Award, Botanical Society of America 1999-2004 Ohio University Presidential Research Scholar in Biomedical and Life Sciences 1993 Edgar T. Wherry Award, Botanical Society of America 1991-1992 Outstanding Graduate Faculty Award, Ohio University 1982-1983 Chairman, Paleobotanical Section, Botanical Society of America 1972-1973 University of Alberta Dissertation Fellow 1971 Paleobotanical (Isabel Cookson) Award, Botanical Society of America Positions Held 2011-present Courtesy Professor of Botany and Plant Pathology, Oregon State University 2008-2009 Visiting Senior Researcher, University of Alberta 2004-present Edwin and Ruth Kennedy Distinguished Professor of Environmental and Plant Biology, Ohio -

Transformative Paleobotany
Chapter 6 Lower Permian Flora of the Sanzenbacher Ranch, Clay County, Texas William A. DiMichele1, Robert W. Hook2, Hans Kerp3, Carol L. Hotton1,4, Cindy V. Looy5 and Dan S. Chaney1 1NMNH Smithsonian Institution, Washington, DC, United States; 2The University of Texas at Austin, Austin, TX, United States; 3Westfälische Wilhelms-Universität Münster, Münster, Germany; 4National Institutes of Health, Bethesda, MD, United States; 5University of California Berkeley, Berkeley, CA, United States 1. INTRODUCTION 1985; Broutin, 1986; Popa, 1999; Steyer et al., 2000; Wagner and Mayoral, 2007; Bercovici and Broutin, 2008; Since 1989, field parties supported by the U.S. National Barthel, 2009; Wagner and Álvarez-Vázquez, 2010; Museum of Natural History have obtained large collections Barthel and Brauner, 2015). Furthermore, because this of mainly Permian plant fossils from north central Texas. locality was collected on three occasions over a time period This work was undertaken to study known localities and to of 50 years and by different parties, comparative analysis of find new fossiliferous deposits that would contribute to a the Sanzenbacher collections provides a basis for assessing better understanding of floral and paleoenvironmental sites that have comparable histories. changes within the region during the early Permian. From the outset, the effort was interdisciplinary and grew, through the contributions of nearly 20 paleobotanists, 2. GEOLOGY palynologists, invertebrate and vertebrate paleontologists, Clay County is the only county in the Permo-Carboniferous and sedimentary geologists of several subdisciplines, to be outcrop belt of north central Texas that lacks marine rocks. quite comprehensive. Our reporting of results, however, has These alluvial sediments accumulated east of a broad been influenced by unexpected developments, including the coastal plain that bordered the Eastern Shelf of the Midland discovery of new plant-fossil assemblages in areas once Basin.