1 CHAPTER 1 INTRODUCTION 1.1 Research Background
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
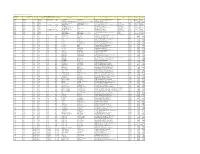
Colgate Palmolive List of Mills As of June 2018 (H1 2018) Direct
Colgate Palmolive List of Mills as of June 2018 (H1 2018) Direct Supplier Second Refiner First Refinery/Aggregator Information Load Port/ Refinery/Aggregator Address Province/ Direct Supplier Supplier Parent Company Refinery/Aggregator Name Mill Company Name Mill Name Country Latitude Longitude Location Location State AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora Agroaceite Extractora Agroaceite Finca Pensilvania Aldea Los Encuentros, Coatepeque Quetzaltenango. Coatepeque Guatemala 14°33'19.1"N 92°00'20.3"W AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora del Atlantico Guatemala Extractora del Atlantico Extractora del Atlantico km276.5, carretera al Atlantico,Aldea Champona, Morales, izabal Izabal Guatemala 15°35'29.70"N 88°32'40.70"O AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora La Francia Extractora La Francia km. 243, carretera al Atlantico,Aldea Buena Vista, Morales, izabal Izabal Guatemala 15°28'48.42"N 88°48'6.45" O Oleofinos Oleofinos Mexico Pasternak - - ASOCIACION AGROINDUSTRIAL DE PALMICULTORES DE SABA C.V.Asociacion (ASAPALSA) Agroindustrial de Palmicutores de Saba (ASAPALSA) ALDEA DE ORICA, SABA, COLON Colon HONDURAS 15.54505 -86.180154 Oleofinos Oleofinos Mexico Pasternak - - Cooperativa Agroindustrial de Productores de Palma AceiteraCoopeagropal R.L. (Coopeagropal El Robel R.L.) EL ROBLE, LAUREL, CORREDORES, PUNTARENAS, COSTA RICA Puntarenas Costa Rica 8.4358333 -82.94469444 Oleofinos Oleofinos Mexico Pasternak - - CORPORACIÓN -

Data Utama Negeri I
Main Data Terengganu Main Data Data Utama Negeri i kandungan contents DATA UTAMA NEGERI 21. Penduduk Mengikut Jantina, Isi Rumah dan Tempat Kediaman 2017 01 Main Data Terengganu Population by Sex, Household & Living Quarters 2017 23. 2. Keluasan, Bilangan JKKK, Guna Tanah & Penduduk Mengikut Daerah 2017 Penduduk Mengikut Kumpulan Umur 2017 Population by Age Group 2017 Area, Number of JKKK, Landused and Population by District 2017 24. 3. Keluasan Mengikut Daerah Penduduk Mengikut Kumpulan Etnik 2017 Population by Ethnic 2017 Area by District Main Data Terengganu Main Data 26. 5. Keluasan Tanah Mengikut Mukim 2017 Kadar Pertumbuhan Penduduk Purata Tahunan Average Annual Population Growth Rate Land Area by Mukim 2017 28. Taburan Peratus Penduduk, Keluasan dan Kepadatan Mengikut Daerah 12. Bilangan Kampung Mengikut JKKK Daerah 2017 Percentage Distribution of Population Area And Density by District Number of Village by District JKKK 2017 30. Penduduk Mengikut Strata 13. Gunatanah Mengikut Daerah 2017 Population by Stratum Landused by District 2017 14. Gunatanah Negeri 2017 Landused by State 2017 SUMBER 03 Resources 34. Sumber PENDUDUK 02 Population Resources 16. Data Penduduk Mengikut Negeri Population Data by State GUNATENAGA 04 Manpower Data Utama Negeri 18. Kadar Pertumbuhan Penduduk Purata Tahunan Mengikut Negeri Average Annual Growth Rate by State 36. Penglibatan Tenaga Buruh 19. Anggaran Penduduk Mengikut Daerah Labour Force Participation Estimated Population by District 37. Taburan Gunatenaga Mengikut Industri Manpower Distribution by Industry KELUARAN DALAM NEGERI KASAR 05 Gross Domestic Product 42 Keluaran Dalam Negeri Kasar (KKDNK) Mengikut Sektor (Harga Malar 2010) Gross Domestic Product (GDP) by Sector (Constant Prices 2010) ii kandungan contens PERINDUSTRIAN TERNAKAN 06 Industry 09 Livestock 48. -

ASSESSMENT REPORT Roundtable on Sustainable Palm Oil Certification R S
PT. MUTUAGUNG LESTARI ASSESSMENT REPORT Roundtable on Sustainable Palm Oil Certification R S P O [√] Reduction Scope Certification Name of Management : KERTEH Palm Oil Mill, Felda Global Ventures (M) sdn Bhd Organisation subsidiary of FELDA Plantation Name : FASSB Kerteh Estate, FGVP Semaring 01 Estate, Kerteh 01 Estate, Kerteh 02 Estate, Kerteh 03 Estate, Kerteh 04 Estate, Kerteh 05 Estate and Kerteh 06 Estate Location : District of Ketengah Jaya, State of Terengganu, Malaysia Certificate Code : MUTU-RSPO/071 Date of Certificate Issue : 1 September 2015 Date of License Issue : 1 September 2015 Date of Certificate Expiry : 31 August 2020 Date of License Expiry : 31 August 2016 Assessment PT. Mutuagung Lestari Reviewed Approved Assessment Date Auditor by by ST-1 8 August 2014 Mahaswaran Maliyapan Mohan Thavarajah, Mohd Hairimi Ganapathy Taufik ST-2 26 – 30 October 2014 Mohd Ali, Nizam Abu Bakar, Ramasamy Margani Dinesh Nadarajah Reduction of Octo HPN Tony 18 November 2015 Taufik Margani Audit Scope Nainggolan Arifiarachman Assessment Approved by MUTUAGUNG LESTARI on: ST-2 7 December 2015 PT Mutuagung Lestari • Raya Bogor Km 33,5 Number 19 • Cimanggis • Depok 16953 • Indonesia Telephone (+62) (21) 8740202 • Fax (+62) (21) 87740745/6 • Email: [email protected] • www.mutucertification.com MUTU Certification Accredited by Accreditation Services International on March 12th, 2014 with registration number RSPO-ACC-007 PT. MUTUAGUNG LESTARI ASSESSMENT REPORT TABLE OF CONTENT FIGURE Figure 1. Location Map of Kerteh Complex 2 Figure 2 Operational -

Terengganu Bilangan Pelajar Bilangan Pekerja Luas Kaw. Sekolah
MAKLUMAT ZON UNTUK TENDER PERKHIDMATAN KEBERSIHAN BANGUNAN DAN KAWASAN BAGI KONTRAK YANG BERMULA PADA 1 JAN 2016 HINGGA 31 DIS 2018 Negeri : Terengganu ENROLMEN KELUASAN PENGHUNI BILANGAN MURID KAWASAN ASRAMA KESELURUHAN Bilangan Bilangan Luas Kaw. Bilangan Bil. Penghuni Bilangan BIL NAMA DAERAH NAMA ZON BIL NAMA SEKOLAH PEKERJA Pelajar Pekerja Sekolah (Ekar) Pekerja Asrama Pekerja (a) (b) (c) (a+b+c) 1 SK DARAU 372 3 2.97 2 5 2 SK TANAH MERAH 377 3 7.98 2 5 3 SK LUBUK KAWAH 654 4 10.50 2 6 4 SK ALOR KELADI 354 3 6.72 2 5 1 BESUT ZON 1 5 PKG SERI PAYONG 1 1 1 2 6 SMK BUKIT PAYONG (Sek & Asrama) 1315 8 16.16 3 300 2 13 7 KIP SK DARAU 1.00 1 1 8 KIP SMK BUKIT PAYONG 2 1 1 JUMLAH PEKERJA KESELURUHAN 38 1 SK BETING LINTANG 211 2 2.13 1 3 2 SK GONG BAYOR 553 4 4.98 1 5 3 SK TEMBILA 503 4 5.19 2 6 2 BESUT ZON 2 4 SK KELUANG 462 3 5.36 2 5 5 SK TENGKU MAHMUD 1043 6 7.61 2 8 6 SMK TEMBILA (Sek & Asrama) 495 3 35.10 5 200 2 10 7 KIP SMK TEMBILA 2.00 1 1 JUMLAH PEKERJA KESELURUHAN 38 1 SK KUALA KUBANG 115 2 4.94 1 3 2 SK JABI 514 4 4.94 1 5 3 SK FELDA SELASIH 110 2 7.91 2 4 4 SK BUKIT TEMPURONG 336 3 5.24 2 5 3 BESUT ZON 3 5 SK APAL 376 3 7.91 2 5 6 SK KERANDANG 545 4 6.92 2 6 7 SK OH 151 2 7.83 2 4 ENROLMEN KELUASAN PENGHUNI 3 BESUT ZON 3 BILANGAN MURID KAWASAN ASRAMA KESELURUHAN Bilangan Bilangan Luas Kaw. -

Important Discovery of Late Early Permian Limestone in Southern Terengganu, Peninsular Malaysia
CEOSE;] '98 Proce erJill.IJ,J, Ceo!. So c. 111a !ay,u'{/ Bu!!. -15, December J999; pp . 455-460 Ninth Regional Congress on Geology, Mineral and GEOSEA '98 Energy Resources of Southeast Asia - GEOSEA '98 17 - 19 August 1998 • Shangri-La Hotel, Kuala Lumpur, Malaysia Important discovery of late Early Permian limestone in southern Terengganu, Peninsular Malaysia 3 HENRI FONTAINEl , IBRAHIM BIN AMNAN2 AND DANIEL V ACHARD 18 Allee de la Chapelle 92140 Clamart, France 2Geological Survey of Malaysia Jalan Sultan Azlan Shah, 31400 Ipoh, Perak, Malaysia 3Paleontologie, Sciences de la Terre, Universite de Lille 59655 Villeneuve d'Ascq cedex, France Abstract: A limestone which has been uncovered during the extension of an oil palm plantation appears to be an important deposit. It is rich in relatively well preserved fossils although it out crops only 500 meters from a granite. The fossils are diverse and consist of common Tubiphytes, a few algae, calcispherids, smaller foraminifers, abundant fusulinaceans (including Levenella, Pamirina, Brevaxina, Chalaroschwagerina, Leeina, Toriyamaia, Laosella), calcareous sponges, a few bryozoans, brachiopods, bivalves, rare gastropods, ostracods and crinoids. They indicate a Late Cisuralian age (Yahtashian Bolorian) and appear to belong to three biozones. The rocks of the area were previously considered to be Early Carboniferous in age. The limestone is commonly a packstone or a wackestone, very rarely a grainstone. Depositional environment was shallow marine. Dolomite is almost absent according to the study of thin sections and the results of chemical analyses. In Terengganu State, north of Seri Bandi in the area of Sungai Patang (a small tributary of Sungai Tebak) about 86 km far from Kuantan and 119 km far from Kuala Terengganu, the extension of Ladang Ketengah Jaya (Ladang = Plantation) has led to the digging of trenches to drain the water away from a swampy area. -

Geochemistry of Sediment in the Major Estuarine Mangrove Forest of Terengganu Region, Malaysia
American Journal of Applied Sciences 5 (12): 1707-1712, 2008 ISSN 1546-9239 © 2008 Science Publications Geochemistry of Sediment in the Major Estuarine Mangrove Forest of Terengganu Region, Malaysia 1B.Y. Kamaruzzaman, 2M.C. Ong, 2M.S. Noor Azhar, 1S. Shahbudin and 1K.C.A. Jalal 1Institute of Oceanography and Maritime Studies, International Islamic University Malaysia, 25200 Kuantan, Pahang, Malaysia 2Institute of Oceanography, Universiti Malaysia Terengganu, 21030 Kuala Terengganu, Terengganu, Malaysia Abstract: Surface sediments collected from seven estuarine mangrove forests of Terengganu region (100 sampling points) were anaylzed for Pb, Cu and Zn using the sensitive Inductively Coupled Plasma Mass Spectrometer (ICP-MS). The average concentration of Pb, Cu and Zn were 10.5±7.12 µg g−1 dry weights, 31.1±16.5 µg g−1 dry weights and 20.8±13.3 µg g−1 dry weights, respectively. The statistical analysis of Pearson correlation matrix has proved that there is a significant relationship between the metal concentration and the grain size. The concentration of Pb, Cu and Zn decreased with the decrease of mean size particle, suggesting their association with the fine fraction of the sediments. In this study, Enrichment Factors (EF) were calculated to assess whether the concentrations observed represent background or contaminated levels. The analysis suggests that all studied elements were considered to be dominantly terrigenous in origin. Data obtained also provides a scientific discovery and data for a better understanding and proper management of the mangrove forests of Terengganu. Key words: Surface sediment, lead, copper, zinc, mangrove forest, enrichment factor INTRODUCTION effects associated with the new developments. -
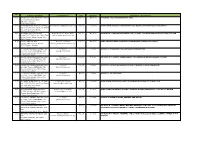
No Company & Address Contact Person Year Duration Description
No Company & Address Contact Person Year Duration Description / Experiences 211 REPSOL OIL & GAS MALAYSIA LIMITED May-17 On going PURCHASE OF JOHN CRANE SHAFT SEAL Level 39, Menara Citibank 165, Jalan Ampang 50450 Kuala Lumpur 210 EP ENGINEERING SDN BHD / REPSOL Anis Adila [email protected] Mar-17 2 months SUPPLY NEW SB252B/4 SUBMERSIBLE SEA WATER LIFT PUMP PARTS FOR REPSOL Unit 203,205 & 206, 2nd Floor, Wisma TKT, 2/4 Jalan Dang Wangi, 50100 Kuala Lumpur 209 PETRONAS Dagangan Berhad Engku M Na'im C Engku Husin Mar-17 On going PROVISION TO REPAIR/REFURBISH ONE (1) UNIT OF A-6893 KRAL ADDITIVE PUMP FOR PDB Melaka Fuel Terminal, Lot 1396-1399 [email protected] Mukim Sungai Udang, Tangga Batu, 76300 Melaka 208 Malaysia LNG Sdn Bhd M Fatihee M Sanasi Feb-17 On going MLNG SATU End Flash Compressor KT5410 Steam Turbine Rotor Repair Tanjung Kidurong P. O. Box 89 [email protected] 97007 Bintulu, Sarawak 207 TECHNIP/PETRONAS FLOATING LNG1 Yi Jia Koo Jan-17 1 month SUPPLY OF STANDPIPE FOR AGIP LEVEL TRANSMITTER Level 51, Tower 2, PETRONAS Twin [email protected] Towers, Kuala Lumpur City Centre, 50088 Kuala Lumpur 206 TECHNIP/PETRONAS FLOATING LNG1 Yi Jia Koo Dec-16 2 weeks PROVISION TO SUPPLY SPARE PARTS FOR JOWA BILGE WATER SEPARATOR PUMP Level 51, Tower 2, PETRONAS Twin [email protected] Towers, Kuala Lumpur City Centre, 50088 Kuala Lumpur 205 TECHNIP/PETRONAS FLOATING LNG1 Yi Jia Koo Nov-16 1 month SUPPLY OF PIPING MATERIALS FOR AIR COMPRESSOR SKID DRAIN LINE Level 51, Tower 2, PETRONAS Twin [email protected] Towers, -

Research Article Detection of Shoreline Changes at Kerteh Bay
©Journal of Applied Sciences & Environmental Sustainability (JASES) 1 (3): 12-20, 2013 Research Article Detection of shoreline changes at Kerteh Bay, Terengganu Malaysia remotely Lawal Abdul Qayoom Tunji, AP Mustafa Hashim Ahmad and AP Khamarulzaman Wan Yusuf Department of Civil Engineering, Universiti Teknologi PETRONAS Seri Iskandar, Tronoh 31750, Perak, Malaysia Email: [email protected] Tel +6 014 923 6294 ARTICLE INFO A b s t r a c t Erosion has been a problem at the Kerteh Bay Coast of Malaysia for over a Article history Received: 27/08/2013 decade. Installation of three submerged breakwaters was put in place to Accepted: 04/11/2013 mitigate the erosion problem in 1996. But unfortunately the projects has not been monitored to ascertain how well the problem has been mitigated. Actual field monitoring only commenced in 2011 after about 14 years. This paper Coastal erosion, remote discuses the performance of the installed submerged breakwater being Sensing, tidal correction monitored using a waterline technique of remote sensing. Satellite imageries of the study area of years, 2006, 2008 and 2009 were acquired for the purpose of monitoring. © Journal of Applied Sciences & Environmental Sustainability. All rights reserved. 1. Introduction Coastal erosion is a global problem; at least 70% of sandy beaches around the world are recessional (Bird,1985). Approximately 86% of U.S East Coast barrier beaches (excluding evolving spit areas) have experienced erosion during the past 100 years (Galgano et al., 2004).Widespread erosion is also well documented in California (Moore et al., 1999) and in the Gulf of Mexico (Morton and McKenna, 1999). -
Codonoboea (Gesneriaceae) in Terengganu, Peninsular Malaysia, Including Three New Species
A peer-reviewed open-access journal PhytoKeys 131: 1–26 (2019) Codonoboea in Terengganu 1 doi: 10.3897/phytokeys.131.35944 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Codonoboea (Gesneriaceae) in Terengganu, Peninsular Malaysia, including three new species Ruth Kiew1, Chung-Lu Lim1 1 Forest Research Institute Malaysia, 52109 Kepong, Selangor, Malaysia Corresponding author: Ruth Kiew ([email protected]) Academic editor: Eric Roalson | Received 6 May 2019 | Accepted 29 July 2019 | Published 2 September 2019 Citation: Kiew R, Lim C-L (2019) Codonoboea (Gesneriaceae) in Terengganu, Peninsular Malaysia, including three new species. PhytoKeys 131: 1–26. https://doi.org/10.3897/phytokeys.131.35944 Abstract Of the 92 Codonoboea species that occur in Peninsular Malaysia, 20 are recorded from the state of Tereng- ganu, of which 9 are endemic to Terengganu including three new species, C. norakhirrudiniana Kiew, C. rheophytica Kiew and C. sallehuddiniana C.L.Lim, that are here described and illustrated. A key and checklist to all the Terengganu species are provided. The majority of species grow in lowland rain forest, amongst which C. densifolia and C. rheophytica are rheophytic. Only four grow in montane forest. The flora of Terengganu is still incompletely known, especially in the northern part of the state and in moun- tainous areas and so, with botanical exploration, more new species can be expected in this speciose genus. Keywords Checklist, key, new species, Codonoboea norakhirrudiniana, Codonoboea rheophytica and Codonoboea salle- huddiniana, endemism Introduction The centre of diversity of the genusCodonoboea (Gesneriaceae) is Peninsular Malaysia from where at least 92 species of the 140 named species are known (Lim and Kiew 2014). -

List of Certified Workshops-Final
TERENGGANU: SENARAI BENGKEL PENYAMAN UDARA KENDERAAN YANG BERTAULIAH (LIST OF CERTIFIED MOBILE AIR-CONDITIONING WORKSHOPS) NO NAMA SYARIKAT ALAMAT POSKOD DAERAH/BANDAR TELEFON NAMA & K/P COMPANY NAME ADDRESS POST CODE DISTRICT/TOWN TELEPHONE NAME& I/C 1 HOCK SENG HWA ELECTRICAL & 11B, SIMPANG 3, JERTEH, BESUT 22000 BESUT TEL : 09-6972168 LAU ENG CHIANG ACCESSORIES 720812-11-5539 2 INSTITUT KEMAHIRAN MARA JALAN BT. TUMBUH, ALOR LINTANG, 22200 BESUT TEL: 09-6957244 MIOR ROSDI BIN YUSOFF BESUT EXT 218 700929-08-5929 3 MARA INST. KEMAHIRAN MARA, JALAN BT. 22200 BESUT TEL: 09-6957244 MOHD. HILMIN BIN TUMBUH, ALOR LINTANG, 22200 BESUT, MAHMOOD 760915- TERENGGANU 03-5589 4 CB DRAGON CAR COOLER & 104-A, JALAN BARU PAK SABAH, 23000 DUNGUN TEL : 09-8482261 CHANG CHEE WEI ACCESSORIES DUNGUN 760617-11-5169 5 KEE AUTO ACCESSORIES & AIR 395-B, JALAN PAKA, BATU 48, DUNGUN 23000 DUNGUN TEL : 09-8442227 KEE CHUNG KEAT CONDITIONS SERVICE 761225-08-6455 1 TERENGGANU: SENARAI BENGKEL PENYAMAN UDARA KENDERAAN YANG BERTAULIAH (LIST OF CERTIFIED MOBILE AIR-CONDITIONING WORKSHOPS) NO NAMA SYARIKAT ALAMAT POSKOD DAERAH/BANDAR TELEFON NAMA & K/P COMPANY NAME ADDRESS POST CODE DISTRICT/TOWN TELEPHONE NAME& I/C 6 MNYJ AC ENTERPRISE LOT 6068, BATU 5, JALAN PAKA, 23000 23000 DUNGUN H/P : 012-9283279 MOHD NAJMUDDIN BIN DUNGUN, TERENGGANU. JUSOH 790203- 11-5377 7 S'DUN AIR-COND SERVICE NO. 171-C, BATU 4½, JALAN PAKA, 23000 DUNGUN H/P : 019-9830318 SARIDUN BIN MOHD NOR DUNGUN, 23000 TERENGGANU DARUL 750114-11-5651 IMAN. 8 UNICO & ACC. LOT 1256 B&C, JALAN BESAR, PAKA, -

Rate and Service Guide Daily Rates Malaysia Effective July 11, 2021 1
2021 UPS® Domestic Rate and Service Guide Daily Rates Malaysia Effective July 11, 2021 1 Area of Service Area of Service West Malaysia – Area of Service within Peninsular FEDERAL TERRITORY Sungai Rambai Temangan Intan Banting Kuala Lumpur Sungai Udang Tanah Merah Jeram Batang Berjuntai Labuan Tanjong Kling Tumpat Kampar Batang Kali Putrajaya Kampong Gajah Batu 9 Cheras NEGERI SEMBILAN PAHANG Kampong Kepayang Batu Arang JOHOR Seremban Kuantan Kamunting Batu Caves Johor Bahru Bahau Bandar Pusat Jengka Kuala Kangsar Beranang = Ayer Hitam Bandar Baru Serting Benta Kuala Kurau Behrang Bakri Batu Kikir Bentong Kuala Sepetang Bukit Rotan Batu Anam Gemas Cameron Highlands Lahat Cyberjaya Batu Pahat Gemencheh Genting Highlands Lambor Kanan Dengkil Bekok Johol Jerantut Langkap Hulu Langat Benut Juasseh Karak Lumut Jenjarom Bukit Gambir Kuala Pilah Kuala Lipis Maliam Nawar Jeram Bukit Pasir Labu Kuala Rompin Mamban Diawan Kajang Chaah Lenggang Lanchang Manong Kapar Endau Linggi Maran Matang Kerling Gelang Patah Mantin Mentakab Menglembu Klang Gerisik Nilai Pekan Padang Rengas KLIA Jementah Pedas Raub Pangkor Kota Kemuning Kahang Port Dickson Tanah Rata Pantai Remis Kuala Kubu Bahru Kg Kenangan Tun Dr Ismail Rembau Temerloh Parit Kuala Selangor Kluang Rompin Parit Buntar Pelabuhan Klang Kota Tinggi Seri Menanti PENANG Pengkalan Hulu Petaling Jaya Kukup Siliau Pulau Pinang Sauk Puchong Kulai Titi Ayer Itam Selama Pulau Carey Labis Balik Pulau Selekoh Pulau Indah Layang-Layang KEDAH Batu Ferringghi Seri Manjong Pulau Ketam Masai Alor Setar Batu Maung -

Beautiful Terengganu Guide Book 2021
Selamat Datang ke Terengganu Negeri Terengganu terletak di Pantai Timur Semenanjung Malaysia dan menghadap Laut China Selatan. Kaya dengan kepelbagaian dan keunikan destinasi serta produk pelancongan yang menarik, kehijauan alam semula jadi yang memayungi bumi Terengganu, kejernihan air laut dan pantai yang mempesonakan, disulami adat dan seni budaya yang sehingga kini menjadi warisan masyarakatnya yang tinggi nilai budi bahasa. Terdapat lapan buah daerah iaitu Kuala Terengganu, Kemaman, Dungun, Besut, Hulu Terengganu, PULAU PERHENTIAN Marang, Setiu dan Kuala Nerus dengan populasi 5TH BEST BEACH TO SWING 13TH BEST BEACH IN THE WORLD penduduk melebihi 1.2 juta. THE HAMMOCK IN THE WORLD 2013: Chosen by CNN The Lonely Planet Bertemakan “Beautiful Terengganu Malaysia”, Travel Book 2010 sub-sektor pelancongan Negeri Terengganu dibahagikan kepada lima sektor utama iaitu Kuala Terengganu, Alam Semula jadi, Pulau dan TERENGGANU PULAU TENGGOL TERENGGANU DRAWBRIDGE TERENGGANU DRAWBRIDGE Pantai, Tasik Kenyir, Seni Budaya dan Warisan. THE BEST DIVING IN PULAU LANG TENGAH DRAWBRIDGE BESTMALDIVES NEW OF TOURISM SOUTHEST ICON ASIA BEST NEW TOURISM ICON PENINSULAR MALAYSIA BEST NEW TOURISM ICON Dengan kepelbagaian dan keunikan sumber alam, MalaysiaThe Asian Tourism Diver CouncilMagazine 2019 Malaysia Tourism Council 2019 The Asian Diver Magazine Malaysian Tourism Council destinasi, produk, keindahan seni budaya warisan Gold Awards dan keenakan makanan yang ada, pastinya TERENGGANU menjadi destinasi wajib CANDAT SOTONG anda kunjungi. ANUGERAH STANDARD