Aminophylline Catalog Number A1755 Storage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Aminophylline on Diaphragmatic Contractility in the Piglet
003 1-3998/90/2803-0196$02.00/0 PEDIATRIC RESEARCH Vol. 28, No. 3, 1990 Copyright 0 1990 International Pediatric Research Foundation, Inc. Printed in (I.S.A. Effect of Aminophylline on Diaphragmatic Contractility in the Piglet DENNIS E. MAYOCK, THOMAS A. STANDAERT, JON F. WATCHKO, AND DAVID E. WOODRUM1 University of Washington School of Medicine, Department of Pediatrics, Division of Neonatal and Respiratory Diseases, Seattle, Washington 98195 ABSTRACT. Minute ventilation, arterial blood gases, ar- of arterial oxygen > 8 kPa (60 torr) in room air, and a partial terial pH, cardiac output, and transdiaphragmatic force pressure of the arterial carbon dioxide 5 6.7 kPa (50 torr) were generation, both during spontaneous ventilation and in accepted for study. The animals were anesthetized with an i.v. response to phrenic nerve stiwulation during airway occlu- combination of chloralose (30 mg/kg) and urethane (1 50 mg/ sion at end expiration, were measured in nine anesthetized, kg) and studied in the supine position. Subsequent infusions of tracheostomized piglets before and 30 min after parenteral anesthetic were used if the piglet developed jaw clonus. A trache- infusion of 20 mg/kg aminophylline. Serum theophylline ostomy was placed and connected to a two-way nonrebreathing levels averaged 109 f 21 ~mol/L(19.7 f 3.7 ~g/mL)at valve (model 2384, Hans Rudolph, Inc., Kansas City, MO). 30 min postinfusion. No significant changes were noted in Inspiratory flow was detected by a hot-wire anemometer and pH, blood gases, blood Pressure, or ventilatory measures integrated to provide tidal volume. All animals breathed 50% after aminophylline. -

Rosemary)-Derived Ingredients As Used in Cosmetics
Safety Assessment of Rosmarinus Officinalis (Rosemary)-Derived Ingredients as Used in Cosmetics Status: Tentative Amended Report for Public Comment Release Date: March 28, 2014 Panel Meeting Date: June 9-10, 2014 All interested persons are provided 60 days from the above release date to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Director, Dr. Lillian J. Gill. The 2014 Cosmetic Ingredient Review Expert Panel members are: Chairman, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, D.P.A. This safety assessment was prepared by Monice M. Fiume, Assistant Director/Senior Scientific Analyst. © Cosmetic Ingredient Review 1620 L Street, NW, Suite 1200♢ Washington, DC 20036 ♢ ph 202.331.0651 ♢ fax 202.331.0088 ♢ [email protected] TABLE OF CONTENTS Abstract ...................................................................................................................................................................................................................................... -

Download Product Insert (PDF)
PRODUCT INFORMATION Proxyphylline Item No. 20937 CAS Registry No.: 603-00-9 Formal Name: 3,7-dihydro-7-(2-hydroxypropyl)-1,3- N dimethyl-1H-purine-2,6-dione O N Synonym: NSC 163343 C H N O MF: 10 14 4 3 N FW: 238.2 N Purity: ≥98% O UV/Vis.: λmax: 273, 324 nm Supplied as: A crystalline solid OH Storage: -20°C Stability: As supplied, 2 years from the QC date provided on the Certificate of Analysis, when stored properly Laboratory Procedures Proxyphylline is supplied as a crystalline solid. A stock solution may be made by dissolving the proxyphylline in the solvent of choice. Proxyphylline is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of proxyphylline in ethanol is approximately 1 mg/ml and approximately 10 mg/ml in DMSO and DMF. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of proxyphylline can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of proxyphylline in PBS, pH 7.2, is approximately 1 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description Proxyphylline is a methylxanthine derivative that has bronchodilatory actions.1 It has also been reported 2 to have vasodilatory and cardiac stimulatory effects. -

Caffeine Versus Aminophylline for Apnea of Prematurity: a Randomized Clinical Trial
World Journal of Peri & Neonatology Vol. 2, No. 2, Fall 2019 Original Article http://wjpn.ssu.ac.ir Caffeine versus Aminophylline for Apnea of Prematurity: A Randomized Clinical Trial Mohamad Hosein Lookzadeh 1,2, Elaha Jafari-Abeshoori 2*, Mahmood Noorishadkam 1,2, Seyed Reza Mirjalili 1,2, Hamid Reza Mohammadi 3, Fatemeh Emambakhshsani 2 1 Department of Pediatrics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 2 Mother and Newborn Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 3 Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran Received: 04 April 2020 Revised: 16 June 2020 Accepted: 20 July 2020 ARTICLE INFO ABSTRACT Corresponding author: Background: Apnea of prematurity is often found in preterm neonates Elaha Jafari-Abeshoori with gestational age less than 34-37 weeks or birth weight (BW) less than 1000 grams. The American Academy of Pediatrics defines apnea as a Email: [email protected] respiratory halt lasting at least 20 seconds, with bradycardia or cyanosis. Methylxanthines reduce the incidence of apnea. The purpose of this study Keywords: was to compare the effect of caffeine and aminophylline on the incidence Aminophylline, of the apnea in premature infants. Caffeine, Methods: This randomized clinical trial study was conducted on 80 Apnea, premature neonates at Shahid Sadoughi hospital in Yazd. The first group Prematurity received the initial dose of 5 mg/kg aminophylline diluted in 5% dextrose with a maintenance dose of 2 mg/kg every 8 hours, while the second group received 30 mg/kg of caffeine diluted in 5% dextrose with a 24-hour maintenance dose of 10 mg/kg. -

The Xanthines (Theobromine and Aminophyllin)
effect of this might conceivably escape detection in THE XANTHINES (THEOBROMINE AND individuals engaged in very heavy work. From this AMINOPHYLLINE) IN THE TREAT- point of view our subjects were particularly favorable for this since of as MENT OF CARDIAC PAIN study, most them, shown in the table, were not engaged in any occupation. HARRY M.D. GOLD, GLYCERYL TRINITRATE TEST NATHANIEL T. M.D. KWIT, Early in the course of the study it was believed AND desirable to HAROLD M.D. restrict the selection of patients to those OTTO, who could establish their qualifications for service in NEW YORK such a study as this by their ability to distinguish An endeavor was made in this study to secure evi- between the efficacy of glyceryl trinitrate taken under dence on the question of whether the xanthines relieve the tongue and a soluble placebo tablet taken in the cardiac pain. same manner for relief during attacks of pain. The SELECTION OF PATIENTS discovery of several patients who found the two equally effective those who had suffered an of The were 100 ambulant in attend- among attack subjects patients thrombosis and were to thoracic ance at the cardiac in whom the of coronary subject pain clinic, diagnosis on effort led us to abandon this restriction. arteriosclerotic heart disease with cardiac pain was made, in accordance with the nomenclature and criteria The results obtained in sixty patients in whom the the New York Heart Association.1 glyceryl trinitrate test was made are of some interest. adopted by These received trinitrate were selected from a total case load of patients glyceryl tablets, %0o They approxi- or cr 0.4 which were mately 700 patients, representing an average sample /4so grain (0.6 mg.), they of the cardiac clinic several racial directed to take under the tongue at the onset of an population, comprising attack of In of these of groups, both native and born. -

Intravenous Aminophylline Treatment for Migraine
Original Observations and Research Personal Observation: Intravenous Aminophylline Treatment for Migraine Michael Kenyon MD, Barry Phillips MD, Christiaan DeWit MBBCh About the Authors Michael Kenyon (near right) and Barry Phillips (far right) are internists, and Christiaan De Wit is an emergency room physician, all practising at Mills Memorial Hospital, in Terrace, British Columbia. Correspondence may be directed to: [email protected] igraine is common condition, often affecting young inhibitor and adenosine antagonist. It has been shown in Mpatients and causing disruption in the home and dipyridamole (Persantine) MIBI studies that dipyridamole workplace alike. The impact of patients presenting to administration inhibits adenosine deaminase in red-cell emergency room services with intractable headache is membranes, increasing blood levels of adenosine. This induces significant, often tying up space and resources in the tedious coronary vasodilation through a low-affinity interaction with wait for a narcotic and sedative “cure.” In Canada alone, 3.2 the A2a receptor. The antidote, aminophylline, preferentially million adults suffer from migraines, and the condition costs binds to this receptor, displacing adenosine and curtailing its the Canadian economy an estimated $500 million annually. effect. 3,4 Absenteeism and loss of productivity resulting from migraines Aminophylline has traditionally and principally been used cost $20 every second. 1 as an intravenously or orally administered bronchodilator in Mills Memorial Hospital is a regional referral centre in asthmatics. Caution in its use should be observed in patients Terrace, British Columbia, serving a population of 70,000 with active peptic ulceration, a low seizure threshold, people. Between June 2011 and January 2012, 21 patients came hypokalemia, tachyarrhythmias, and acute congestive heart to the emergency room (ER) suffering from symptoms failure (CHF). -

Enabling Direct Preferential Crystallization in a Stable Racemic
Enabling Direct Preferential Crystallization in a Stable Racemic Compound System Lina Harfouche, Clément Brandel, Yohann Cartigny, Joop ter Horst, Gérard Coquerel, Samuel Petit To cite this version: Lina Harfouche, Clément Brandel, Yohann Cartigny, Joop ter Horst, Gérard Coquerel, et al.. Enabling Direct Preferential Crystallization in a Stable Racemic Compound System. Molecular Pharmaceutics, American Chemical Society, 2019, 16 (11), pp.4670-4676. 10.1021/acs.molpharmaceut.9b00805. hal- 02331962 HAL Id: hal-02331962 https://hal-normandie-univ.archives-ouvertes.fr/hal-02331962 Submitted on 24 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Enabling Direct Preferential Crystallization in a Stable Racemic Compound System Lina C. Harfouche,† Clément Brandel,†* Yohann Cartigny,† Joop H. ter Horst,‡ Gérard Coquerel,† and Samuel Petit† † Universite de Rouen Normandie, UFR des Sciences et Techniques, Laboratoire SMS-EA3233, Place Emile Blondel, 76821, Mont-Saint-Aignan, France ‡EPSRC Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation (CMAC), Strathclyde Insti- tute of Pharmacy and Biomedical Sciences (SIPBS), Technology and Innovation Centre, University of Strathclyde, 99 George Street, Glasgow G1 1RD (UK) Supporting Information Placeholder 8 ABSTRACT: The preparative resolution by preferential crystal- separation methods, including chiral chromatography and enzy- 9 lization (PC) of proxyphylline has been achieved despite the matic resolution. -

Pjp1'2003.Vp:Corelventura
Copyright © 2003 by Institute of Pharmacology Polish Journal of Pharmacology Polish Academy of Sciences Pol. J. Pharmacol., 2003, 55, 103107 ISSN 1230-6002 SHORT COMMUNICATION INFLUENCE OF LY 300164, AN AMPA/KAINATE RECEPTOR ANTAGONIST UPON THE ANTICONVULSANT ACTION OF ANTIEPILEPTIC DRUGS AGAINST AMINOPHYLLINE-INDUCED SEIZURES IN MICE Mariusz Œwi¹der1, Hubert KuŸniar1, Zdzis³aw Kleinrok1 , Stanis³aw J. Czuczwar2,3,# Department of Pharmacology and Toxicology, Department of Pathophysiology, Medical University, Jaczewskiego 8, PL 20-090 Lublin, !Isotope Laboratory, Institute of Agricultural Medicine, Jaczewskiego 2, PL 20-950 Lublin, Poland Influence of LY 300164, an AMPA/kainate receptor antagonist, upon the anticonvulsant action of antiepileptic drugs against aminophylline-induced seizures in mice. M. ŒWI¥DER, H. KUNIAR, Z. KLEINROK , S.J. CZU- CZWAR. Pol. J. Pharmacol., 2003, 55, 103–107. LY 300164 {7-acetyl-3-(4-aminophenyl)-8,9-dihydro-8-methyl-7H-1,3- dioxazolo[4,5-h] [2,3]-benzodiazepine}, a novel AMPA/kainate receptor an- tagonist, administered intraperitoneally protected mice against aminophyl- line-induced seizures. At doses up to 0.5 mg/kg, which did not significantly affect the convulsant activity of aminophylline, it potentiated the protective activity of diazepam. On the other hand, LY 300164 used at the lowest pro- tective dose of 1.0 mg/kg enhanced anticonvulsant activity of all antiepilep- tic drugs tested in this seizure model. However, LY 300164 neither alone nor combined with antiepileptic drugs, reduced aminophylline-induced mortality. Key words: antiepileptic drugs, LY 300164, aminophylline-induced sei- zures correspondence; e-mail: [email protected] M. Œwi¹der, H. KuŸniar, Z. -
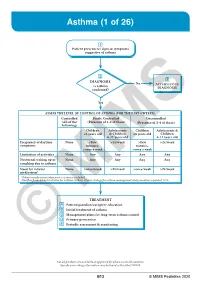
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough -

Persantine® (Dipyridamole USP) 25 Mg, 50 Mg, and 75 Mg Tablets
® Persantine (dipyridamole USP) 25 mg, 50 mg, and 75 mg tablets Rx only Prescribing Information DESCRIPTION PERSANTINE® (dipyridamole USP) is a platelet inhibitor chemically described as 2,2',2'',2'''-[(4,8 Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula: OH N N N N HO N N N OH N OH C24H40N8O4 Mol. Wt. 504.63 Dipyridamole is an odorless yellow crystalline powder, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and practically insoluble in water. PERSANTINE tablets for oral administration contain: Active Ingredient TABLETS 25 mg, 50 mg, and 75 mg: dipyridamole USP 25 mg, 50 mg and 75 mg, respectively. Inactive Ingredients TABLETS 25 mg, 50 mg, and 75 mg: acacia, carnauba wax, corn starch, edible white ink, lactose monohydrate, magnesium stearate, D&C yellow #10 aluminum lake, D&C red #30, helendon aluminum pink lake, sodium benzoate, methylparaben, propylparaben, polyethylene glycol, povidone, sucrose, talc, titanium dioxide, and white wax. CLINICAL PHARMACOLOGY It is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic complications occurring in connection with prosthetic heart valve replacement. PERSANTINE tablets have been found to lengthen abnormally shortened platelet survival time in a dose-dependent manner. In three randomized controlled clinical trials involving 854 patients who had undergone surgical placement of a prosthetic heart valve, PERSANTINE tablets, in combination with warfarin, decreased the incidence of postoperative thromboembolic events by 62 to 91% compared to warfarin treatment alone. -

Physiological Effects of Caffeine and Its Congeners Present in Tea And
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 August 2018 doi:10.20944/preprints201808.0032.v1 1 Type of the paper: Review 2 3 Physiological effects of caffeine and its congeners 4 present in tea and coffee beverages 5 6 I. Iqbal1, M. N. Aftab2, M. A. Safer3, M. Menon4, M. Afzal5⌘ 7 1Department of Life Sciences, Lahore College for Women, Lahore, Pakistan 8 2Institute of Biochemistry and Biotechnology, Government College University, 9 Lahore 54000, Pakistan 10 3Department of Biological Sciences, Faculty of Science, Kuwait University, Kuwait 11 4Plamer University (West Campus) San Jose, CA 12 5Department of Biological Sciences, Faculty of Science, Kuwait University, Kuwait 13 ⌘ Correspondence: [email protected], Tel. +1 352 681 7347 14 15 16 Running title: Caffeine 17 18 19 20 21 22 23 Corresponding author: 24 M. Afzal, 25 10547 NW 14th PL. 26 Gainesville, FL. USA 27 email: [email protected] 28 Tel. +1 352 681 7347 29 30 1 © 2018 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 August 2018 doi:10.20944/preprints201808.0032.v1 31 Abstract: Tea and coffee are the most commonly used beverages throughout the 32 world. Both decoctions are rich in small organic molecules such as 33 phenolics/polyphenolics, purine alkaloids, many methylxanthines, substituted 34 benzoic and cinnamic acids. Many of these molecules are physiologically 35 chemopreventive and chemoprotective agents against many severe conditions such 36 as cancer, Alzheimer, Parkinsonism, inflammation, sleep apnea, cardiovascular 37 disorders, bradycardia, fatigue, muscular relaxation, and oxidative stress. -

Substance Abuse
\1\A-10/PSA/93.10 ORIGINAL: ENGUSH DISTR.: GENERAL PROGRAMME ON SUBSTANCE ABUSE Approaches to Treatment of Substance Abuse WORLD HEALTH ORGANIZATION ABSTRACf The aim of this report is to provide an updated description of different methods used around the world to treat health problems associated with substance use. The scope of treatment approaches currently practised around the world is wide, ranging from traditional healing practices via mutual self-help groups, psychological/behavioural treatment to pharmacological treatment. Also included is a chapter on the generalist responses to alcohol and other drug problems and a chapter on the concept of harm minimization. Another aspect is a chapter on specific descriptors, which distinguishes different treatments from one another and provides economic means of describing them. Social and structural extra-treatment factors influencing the treatment process are also a subject described in this report. ©World Health Organization, 1993 This document is not a formal publication of the World Health Organization (WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated in part or in whole, but not for sale nor for use in conjunction with commercial purposes. The views expressed in documents by named authors are solely the responsibility of those authors. ii CONTENTS page Acknowledgements . v Introduction . 1 I. Descriptors of Treatments . 3 11. Factors Influencing Treatment . 31 Ill. Generalist Responses to Alcohol and other Drug Problems . 53 IV. Pharmacological Treatment of Dependence on Alcohol and other Drugs: An Overview . 75 V. Psychological, Behavioural and Psychodynamic Treatments for Substance Abuse . 103 VI.