REVIEW ARTICLE the Retrosplenial-Parietal Network and Reference Frame Coordination for Spatial Navigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Mechanisms of Navigation Involving Interactions of Cortical and Subcortical Structures
J Neurophysiol 119: 2007–2029, 2018. First published February 14, 2018; doi:10.1152/jn.00498.2017. REVIEW Where Are You Going? The Neurobiology of Navigation Neural mechanisms of navigation involving interactions of cortical and subcortical structures James R. Hinman, Holger Dannenberg, Andrew S. Alexander, and Michael E. Hasselmo Center for Systems Neuroscience, Boston University, Boston, Massachusetts Submitted 5 July 2017; accepted in final form 1 February 2018 Hinman JR, Dannenberg H, Alexander AS, Hasselmo ME. Neural mecha- nisms of navigation involving interactions of cortical and subcortical structures. J Neurophysiol 119: 2007–2029, 2018. First published February 14, 2018; doi: 10.1152/jn.00498.2017.—Animals must perform spatial navigation for a range of different behaviors, including selection of trajectories toward goal locations and foraging for food sources. To serve this function, a number of different brain regions play a role in coding different dimensions of sensory input important for spatial behavior, including the entorhinal cortex, the retrosplenial cortex, the hippocampus, and the medial septum. This article will review data concerning the coding of the spatial aspects of animal behavior, including location of the animal within an environment, the speed of movement, the trajectory of movement, the direction of the head in the environment, and the position of barriers and objects both relative to the animal’s head direction (egocentric) and relative to the layout of the environment (allocentric). The mechanisms for coding these important spatial representations are not yet fully understood but could involve mechanisms including integration of self-motion information or coding of location based on the angle of sensory features in the environment. -
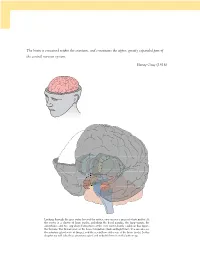
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Reversible Verbal and Visual Memory Deficits After Left Retrosplenial Infarction
Journal of Clinical Neurology / Volume 3 / March, 2007 Case Report Reversible Verbal and Visual Memory Deficits after Left Retrosplenial Infarction Jong Hun Kim, M.D.*, Kwang-Yeol Park, M.D.†, Sang Won Seo, M.D.*, Duk L. Na, M.D.*, Chin-Sang Chung, M.D.*, Kwang Ho Lee, M.D.*, Gyeong-Moon Kim, M.D.* *Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea †Department of Neurology, Chung-Ang University Medical Center, Chung-Ang University School of Medicine, Seoul, Korea The retrosplenial cortex is a cytoarchitecturally distinct brain structure located in the posterior cingulate gyrus and bordering the splenium, precuneus, and calcarine fissure. Functional imaging suggests that the retrosplenium is involved in memory, visuospatial processing, proprioception, and emotion. We report on a patient who developed reversible verbal and visual memory deficits following a stroke. Neuro- psychological testing revealed both anterograde and retrograde memory deficits in verbal and visual modalities. Brain diffusion-weighted and T2-weighted magnetic resonance imaging (MRI) demonstrated an acute infarction of the left retrosplenium. J Clin Neurol 3(1):62-66, 2007 Key Words : Retrosplenium, Memory, Amnesia We report on a patient who developed both verbal INTRODUCTION and visual memory deficits after an acute infarction of the retrosplenial cortex. The main structures related to human memory are the Papez circuit, the basolateral limbic circuit, and the basal forebrain, which communicate with each other through white-matter tracts. Damage to these structures (in- cluding the communication tracts) from hemorrhages, infarctions, and tumors can result in memory dis- turbances.1,2 In addition to these structures, Valenstein et al. -

Changing the Cortical Conductor's Tempo: Neuromodulation of the Claustrum
REVIEW published: 13 May 2021 doi: 10.3389/fncir.2021.658228 Changing the Cortical Conductor’s Tempo: Neuromodulation of the Claustrum Kelly L. L. Wong 1, Aditya Nair 2,3 and George J. Augustine 1,2* 1Neuroscience and Mental Health Program, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore, 2Institute of Molecular and Cell Biology (IMCB), Agency for Science, Technology and Research (A∗STAR), Singapore, Singapore, 3Computation and Neural Systems, California Institute of Technology, Pasadena, CA, United States The claustrum is a thin sheet of neurons that is densely connected to many cortical regions and has been implicated in numerous high-order brain functions. Such brain functions arise from brain states that are influenced by neuromodulatory pathways from the cholinergic basal forebrain, dopaminergic substantia nigra and ventral tegmental area, and serotonergic raphe. Recent revelations that the claustrum receives dense input from these structures have inspired investigation of state-dependent control of the claustrum. Here, we review neuromodulation in the claustrum—from anatomical connectivity to behavioral manipulations—to inform future analyses of claustral function. Keywords: claustrum, acetylcholine, serotonin, dopamine, neuromodulation Edited by: Edouard Pearlstein, INTRODUCTION Independent Researcher, Marseille, France The claustrum is a long and irregular sheet of neurons nestled between the insula and striatum. As it is known to be heavily and bilaterally connected to many brain regions in organisms ranging Reviewed by: Ami Citri, from mice to humans (Sherk, 1986; Torgerson et al., 2015; Wang et al., 2017, 2019; Zingg et al., Hebrew University of Jerusalem, 2018), the claustrum has been likened to a cortical conductor (Crick and Koch, 2005). -

Retrosplenial Cortex Is Necessary for Latent Learning in Mice
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.21.453258; this version posted July 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Retrosplenial cortex is necessary for spatial and non-spatial latent learning in mice Ana Carolina Bottura de Barros1,2*, Liad J. Baruchin1#, Marios C. Panayi3#, Nils Nyberg1, Veronika Samborska1, Mitchell T. Mealing1, Thomas Akam3, Jeehyun Kwag4, David M. Bannerman3 & Michael M. Kohl1,2* 1 Dept. of Physiology, Anatomy and Genetics, University of Oxford, Oxford, OX1 3PT, UK 2 Institute of Neuroscience and Psychology, University of Glasgow, Glasgow, G12 8QQ, UK 3 Dept. of Experimental Psychology, University of Oxford, Oxford, OX1 3SR, UK 4 Dept. of Brain and Cognitive Engineering, Korea University, Seoul, 136-71, Republic of Korea #These authors contributed equally *Correspondence: [email protected] & [email protected] Keywords: Latent Learning, Retrosplenial Cortex, Optogenetics, Internal Model, Mice 1/19 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.21.453258; this version posted July 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Abstract 2 Latent learning occurs when associations are formed between stimuli in the absence of explicit 3 reinforcement. -

The Pre/Parasubiculum: a Hippocampal Hub for Scene- Based Cognition? Marshall a Dalton and Eleanor a Maguire
Available online at www.sciencedirect.com ScienceDirect The pre/parasubiculum: a hippocampal hub for scene- based cognition? Marshall A Dalton and Eleanor A Maguire Internal representations of the world in the form of spatially which posits that one function of the hippocampus is to coherent scenes have been linked with cognitive functions construct internal representations of scenes in the ser- including episodic memory, navigation and imagining the vice of memory, navigation, imagination, decision-mak- future. In human neuroimaging studies, a specific hippocampal ing and a host of other functions [11 ]. Recent inves- subregion, the pre/parasubiculum, is consistently engaged tigations have further refined our understanding of during scene-based cognition. Here we review recent evidence hippocampal involvement in scene-based cognition. to consider why this might be the case. We note that the pre/ Specifically, a portion of the anterior medial hippocam- parasubiculum is a primary target of the parieto-medial pus is consistently engaged by tasks involving scenes temporal processing pathway, it receives integrated [11 ], although it is not yet clear why a specific subre- information from foveal and peripheral visual inputs and it is gion of the hippocampus would be preferentially contiguous with the retrosplenial cortex. We discuss why these recruited in this manner. factors might indicate that the pre/parasubiculum has privileged access to holistic representations of the environment Here we review the extant evidence, drawing largely from and could be neuroanatomically determined to preferentially advances in the understanding of visuospatial processing process scenes. pathways. We propose that the anterior medial portion of the hippocampus represents an important hub of an Address extended network that underlies scene-related cognition, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, and we generate specific hypotheses concerning the University College London, 12 Queen Square, London WC1N 3BG, UK functional contributions of hippocampal subfields. -

Reading Speech from Still and Moving Faces: the Neural Substrates of Visible Speech
Reading Speech from Still and Moving Faces: The Neural Substrates of Visible Speech Gemma A. Calvert1 and Ruth Campbell2 Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/15/1/57/1757731/089892903321107828.pdf by guest on 18 May 2021 Abstract & Speech is perceived both by ear and by eye. Unlike heard regions including the left inferior frontal (Broca’s) area, left speech, some seen speech gestures can be captured in superior temporal sulcus (STS), and left supramarginal gyrus stilled image sequences. Previous studies have shown that in (the dorsal aspect of Wernicke’s area). Stilled speech hearing people, natural time-varying silent seen speech can sequences also generated activation in the ventral premotor access the auditory cortex (left superior temporal regions). cortex and anterior inferior parietal sulcus bilaterally. Using functional magnetic resonance imaging (fMRI), the Moving faces generated significantly greater cortical activa- present study explored the extent to which this circuitry was tion than stilled face sequences, and in similar regions. activated when seen speech was deprived of its time-varying However, a number of differences between stilled and moving characteristics. speech were also observed. In the visual cortex, stilled faces In the scanner, hearing participants were instructed to generated relatively more activation in primary visual regions look for a prespecified visible speech target sequence (‘‘voo’’ (V1/V2), while visual movement areas (V5/MT+) were or ‘‘ahv’’) among other monosyllables. In one condition, the activated to a greater extent by moving faces. Cortical regions image sequence comprised a series of stilled key frames activated more by naturally moving speaking faces included showing apical gestures (e.g., separate frames for ‘‘v’’ and the auditory cortex (Brodmann’s Areas 41/42; lateral parts of ‘‘oo’’ [from the target] or ‘‘ee’’ and ‘‘m’’ [i.e., from Heschl’s gyrus) and the left STS and inferior frontal gyrus. -

Default Mode Network in Childhood Autism Posteromedial Cortex Heterogeneity and Relationship with Social Deficits
ARCHIVAL REPORT Default Mode Network in Childhood Autism: Posteromedial Cortex Heterogeneity and Relationship with Social Deficits Charles J. Lynch, Lucina Q. Uddin, Kaustubh Supekar, Amirah Khouzam, Jennifer Phillips, and Vinod Menon Background: The default mode network (DMN), a brain system anchored in the posteromedial cortex, has been identified as underconnected in adults with autism spectrum disorder (ASD). However, to date there have been no attempts to characterize this network and its involvement in mediating social deficits in children with ASD. Furthermore, the functionally heterogeneous profile of the posteromedial cortex raises questions regarding how altered connectivity manifests in specific functional modules within this brain region in children with ASD. Methods: Resting-state functional magnetic resonance imaging and an anatomically informed approach were used to investigate the functional connectivity of the DMN in 20 children with ASD and 19 age-, gender-, and IQ-matched typically developing (TD) children. Multivariate regression analyses were used to test whether altered patterns of connectivity are predictive of social impairment severity. Results: Compared with TD children, children with ASD demonstrated hyperconnectivity of the posterior cingulate and retrosplenial cortices with predominately medial and anterolateral temporal cortex. In contrast, the precuneus in ASD children demonstrated hypoconnectivity with visual cortex, basal ganglia, and locally within the posteromedial cortex. Aberrant posterior cingulate cortex hyperconnectivity was linked with severity of social impairments in ASD, whereas precuneus hypoconnectivity was unrelated to social deficits. Consistent with previous work in healthy adults, a functionally heterogeneous profile of connectivity within the posteromedial cortex in both TD and ASD children was observed. Conclusions: This work links hyperconnectivity of DMN-related circuits to the core social deficits in young children with ASD and highlights fundamental aspects of posteromedial cortex heterogeneity. -

Are the Neural Correlates of Consciousness in the Front Or in the Back of the Cerebral Cortex?
bioRxiv preprint doi: https://doi.org/10.1101/118273; this version posted March 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence Melanie Boly1,2*, Marcello Massimini3,4, Naotsugu Tsuchiya5, Bradley R. Postle2,6, Christof Koch7, Giulio Tononi2* 1 Department of Neurology, University of Wisconsin, Madison, WI, 53705 USA 2 Department of Psychiatry, University of Wisconsin, Madison, WI, 53719 USA 3 Department of Biomedical and Clinical Sciences ‘Luigi Sacco’, University of Milan, Milan, 20157, Italy 4 Instituto Di Ricovero e Cura a Carattere Scientifico, Fondazione Don Carlo Gnocchi, Milan, 20148, Italy 6 Department of Psychology, Monash University, Melbourne, 3168, Australia 6 Department of Psychology, University of Wisconsin, Madison, WI, 53705 USA 7 Allen Institute for Brain Science, Seattle, WA, 98109 USA Abbreviated title: Is consciousness in the front versus the back of corteX? Correspondence to: Melanie Boly: [email protected] or Giulio Tononi: [email protected]. Address: 6001 Research Park Boulevard, Madison WI 53719 Number of pages: 25 Number of figures: 5 Number of words for Abstract: 45; number of words for Main teXt: 3750 Conflicts of Interest: The authors declare no conflicts of interest. Acknowledgments: This work was supported by NIH/NINDS 1R03NS096379 (to M.B.), by the Tiny Blue Dot Foundation and the Distinguished Chair in Consciousness Science (University of Wisconsin) (to G.T.), and by the James S. -

Robust Vestibular Self-Motion Signals in Macaque Posterior Cingulate Region Bingyu Liu1,2, Qingyang Tian1,2, Yong Gu1,2*
RESEARCH ARTICLE Robust vestibular self-motion signals in macaque posterior cingulate region Bingyu Liu1,2, Qingyang Tian1,2, Yong Gu1,2* 1CAS Center for Excellence in Brain Science and Intelligence Technology, Key Laboratory of Primate Neurobiology, Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China; 2University of Chinese Academy of Sciences, Beijing, China Abstract Self-motion signals, distributed ubiquitously across parietal-temporal lobes, propagate to limbic hippocampal system for vector-based navigation via hubs including posterior cingulate cortex (PCC) and retrosplenial cortex (RSC). Although numerous studies have indicated posterior cingulate areas are involved in spatial tasks, it is unclear how their neurons represent self-motion signals. Providing translation and rotation stimuli to macaques on a 6-degree-of-freedom motion platform, we discovered robust vestibular responses in PCC. A combined three-dimensional spatiotemporal model captured data well and revealed multiple temporal components including velocity, acceleration, jerk, and position. Compared to PCC, RSC contained moderate vestibular temporal modulations and lacked significant spatial tuning. Visual self-motion signals were much weaker in both regions compared to the vestibular signals. We conclude that macaque posterior cingulate region carries vestibular-dominant self-motion signals with plentiful temporal components that could be useful for path integration. Introduction Navigation is a fundamental and indispensable ability for creatures -
Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning
The Journal of Neuroscience, August 15, 2002, 22(16):7288–7296 Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning David Gaffan,1 Alexander Easton,2 and Amanda Parker2 1Department of Experimental Psychology, Oxford University, Oxford OX1 3UD, United Kingdom, and 2School of Psychology, Nottingham University, Nottingham NG7 2RD, United Kingdom Macaque monkeys learned a strategy task in which two groups TSϩAMϩFX cannot be generally attributed to the partial tem- of visual objects needed to be treated differently, one with poral–frontal disconnection that this lesion creates, and there- persistent and one with sporadic object choices, to obtain food fore support the hypothesis that the amnesic effects of this rewards. After preoperative training, they were divided into two lesion are caused primarily by the disconnection of temporal surgical groups of three monkeys each. One group received cortex from ascending inputs from the basal forebrain. The crossed unilateral removals of frontal cortex and inferior tem- results also show that temporal–frontal interaction in strategy poral cortex (IT ϫ FC) and were severely impaired in performing implementation does not require those routes of temporal– the strategy task. The other group received bilateral transection frontal interaction that are interrupted in TSϩAMϩFX, and of anterior temporal stem, amygdala, and fornix (TSϩAMϩFX) therefore support the hypothesis that projections to other pos- and were unimpaired in performing the strategy task. Subse- terior cortical areas allow temporal and frontal cortex to interact quently the same animals were tested in visual object–reward with each other by multisynaptic corticocortical routes in strat- association learning. -

Experience Sampling During Fmri Reveals Default Network and Executive System Contributions to Mind Wandering
Experience sampling during fMRI reveals default network and executive system contributions to mind wandering Kalina Christoffa,1, Alan M. Gordonb, Jonathan Smallwoodc, Rachelle Smitha, and Jonathan W. Schoolerc aDepartment of Psychology, University of British Columbia, Vancouver, BC, Canada V6Z 2R8; bDepartment of Psychology, Stanford University, CA 94305; and cDepartment of Psychology, University of California, Santa Barbara, CA 93106 Edited by Michael I. Posner, University of Oregon, Eugene, OR, and approved March 27, 2009 (received for review February 9, 2009) Although mind wandering occupies a large proportion of our there were no online measures of mind wandering taken during waking life, its neural basis and relation to ongoing behavior scanning, it is possible that the observed default network recruit- remain controversial. We report an fMRI study that used experi- ment could be due to factors other than mind wandering. In this ence sampling to provide an online measure of mind wandering vein, Gilbert et al. (9) recently argued that instead of mind during a concurrent task. Analyses focused on the interval of time wandering, activations in the medial PFC part of the default immediately preceding experience sampling probes demonstrate network may reflect stimulus-related thought such as enhanced activation of default network regions during mind wandering, a watchfulness toward the external environment that is also likely to finding consistent with theoretical accounts of default network occur during highly practiced tasks. functions. Activation in medial prefrontal default network regions A key objective of the present study was to provide a direct was observed both in association with subjective self-reports of empirical test for the hypothesis that default network recruitment, mind wandering and an independent behavioral measure (perfor- including medial PFC regions, occurs during the precise moments mance errors on the concurrent task).