Cortical Connectivity Maps Reveal Anatomically Distinct Areas in the Parietal Cortex of the Rat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
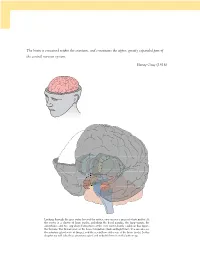
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Representation of Spatial Orientation by the Intrinsic Dynamics of the Head-Direction Cell Ensemble: a Theory
The Journal of Neuroscience, March 15, 1996, 16(6):2112-2126 Representation of Spatial Orientation by the Intrinsic Dynamics of the Head-Direction Cell Ensemble: A Theory Kechen Zhang Department of Cognitive Science, University of California at San Diego, La Jolla, California 92093-05 15 The head-direction (HD) cells found in the limbic system in disturbances to its shape, and the shift speed can be controlled freely moving rats represent the instantaneous head direction accurately by the strength of the odd-weight component. The of the animal in the horizontal plane regardless of the location generic formulation of the shift mechanism is determined of the animal. The internal direction represented by these cells uniquely within the current theoretical framework. The attractor uses both self-motion information for inet-tially based updating dynamics of the system ensures modality-independence of the and familiar visual landmarks for calibration. Here, a model of internal representation and facilitates the correction for cumu- the dynamics of the HD cell ensemble is presented. The sta- lative error by the putative local-view detectors. The model bility of a localized static activity profile in the network and a offers a specific one-dimensional example of a computational dynamic shift mechanism are explained naturally by synaptic mechanism in which a truly world-centered representation can weight distribution components with even and odd symmetry, be derived from observer-centered sensory inputs by integrat- respectively. Under symmetric weights or symmetric reciprocal ing self-motion information. connections, a stable activity profile close to the known direc- Key words: head-direction cell; spatial orientation; attractor tional tuning curves will emerge. -

The Brain Compass: a Perspective on How Self-Motion Updates the Head Direction Cell Attractor
bioRxiv preprint doi: https://doi.org/10.1101/189464; this version posted September 17, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The brain compass: a perspective on how self-motion updates the head direction cell attractor Jean Laurens, Dora E. Angelaki Dept. of Neuroscience, Baylor College of Medicine, Houston, TX, 77030, USA This study was supported by National Institutes of Health grants R21 NS098146 and R01- DC004260. Address for correspondence: Angelaki, Dora Department of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 Office: (713) 798-1469 E-mail: [email protected] ABSTRACT Head Direction cells form an internal compass that signals head azimuth orientation even in the absence of visual landmarks. It is well accepted that head direction properties are generated through a ring attractor that is updated using rotation self-motion cues. The properties and origin of this self-motion velocity drive remain, however, unknown. We propose a unified, quantitative framework whereby the attractor velocity input represents a multisensory self-motion estimate computed through an internal model that uses sensory prediction error based on vestibular, visual, and somatosensory cues to improve on-line motor drive. We show how context- dependent strength of recurrent connections within the attractor itself, rather than the self- motion input, explain differences in head direction cell firing between free foraging and restrained movements. We also summarize recent findings on how head tilt relative to gravity influences the azimuth coding of head direction cells, and explain why and how these effects reflect an updating self-motion velocity drive that is not purely egocentric. -

Head Direction Cells: Properties and Functional Significance Robert U Mullet-L, James B Ranck Jr* and Jeffrey S Taubea
196 Head direction cells: properties and functional significance Robert U Mullet-l, James B Ranck Jr* and Jeffrey S Taubea The strong signal carried by head direction cells in the Under all known circumstances, postsubicular HD cells, postsubiculum complements the positional signal carried the first class to be discovered, discharge only as a by hippocampal place cells; together, the directional and function of the direction in which the rat’s head points positional signals provide the information necessary to permit in the horizontal plane, independent of head location. rats to generate and carry out intelligent, efficient solutions Postsubicular HD cell discharge is rapid only when the to spatial problems. Our opinion is that the hippocampal head points in an -90” sector of headings centered on a positional system acts as a cognitive map and that the role ‘preferred direction’. The relationship between direction of the directional system is to put the map into register with and discharge rate is steep; the function is triangular, the environment. In this way, paths found using the map can so that firing rate decreases linearly in both directions be properly executed. Head direction cells have recently been away from the preferred direction, to be nearly zero when discovered in parts of the thalamus reciprocally connected 45” away from the peak [2,3’]. In contrast to place cells, with the postsubiculum; such cells provide important clues to the reliability of postsubicular HD cell firing is very the organization of the directional system. high. (Some of the properties of HD cells are shown in Figure 2c.) Addresses tg2Department of Physiology, SUNY Health Science Center at In this review, we will first briefly recapitulate what is Brooklyn, 450 Clarkson Avenue, Brooklyn, New York 11203, USA known about HD cells (also called directional cells), t e-mail: [email protected] and then summarize what has been learned about them se-mail: [email protected] during the past two years. -

Reading Speech from Still and Moving Faces: the Neural Substrates of Visible Speech
Reading Speech from Still and Moving Faces: The Neural Substrates of Visible Speech Gemma A. Calvert1 and Ruth Campbell2 Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/15/1/57/1757731/089892903321107828.pdf by guest on 18 May 2021 Abstract & Speech is perceived both by ear and by eye. Unlike heard regions including the left inferior frontal (Broca’s) area, left speech, some seen speech gestures can be captured in superior temporal sulcus (STS), and left supramarginal gyrus stilled image sequences. Previous studies have shown that in (the dorsal aspect of Wernicke’s area). Stilled speech hearing people, natural time-varying silent seen speech can sequences also generated activation in the ventral premotor access the auditory cortex (left superior temporal regions). cortex and anterior inferior parietal sulcus bilaterally. Using functional magnetic resonance imaging (fMRI), the Moving faces generated significantly greater cortical activa- present study explored the extent to which this circuitry was tion than stilled face sequences, and in similar regions. activated when seen speech was deprived of its time-varying However, a number of differences between stilled and moving characteristics. speech were also observed. In the visual cortex, stilled faces In the scanner, hearing participants were instructed to generated relatively more activation in primary visual regions look for a prespecified visible speech target sequence (‘‘voo’’ (V1/V2), while visual movement areas (V5/MT+) were or ‘‘ahv’’) among other monosyllables. In one condition, the activated to a greater extent by moving faces. Cortical regions image sequence comprised a series of stilled key frames activated more by naturally moving speaking faces included showing apical gestures (e.g., separate frames for ‘‘v’’ and the auditory cortex (Brodmann’s Areas 41/42; lateral parts of ‘‘oo’’ [from the target] or ‘‘ee’’ and ‘‘m’’ [i.e., from Heschl’s gyrus) and the left STS and inferior frontal gyrus. -

Are the Neural Correlates of Consciousness in the Front Or in the Back of the Cerebral Cortex?
bioRxiv preprint doi: https://doi.org/10.1101/118273; this version posted March 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence Melanie Boly1,2*, Marcello Massimini3,4, Naotsugu Tsuchiya5, Bradley R. Postle2,6, Christof Koch7, Giulio Tononi2* 1 Department of Neurology, University of Wisconsin, Madison, WI, 53705 USA 2 Department of Psychiatry, University of Wisconsin, Madison, WI, 53719 USA 3 Department of Biomedical and Clinical Sciences ‘Luigi Sacco’, University of Milan, Milan, 20157, Italy 4 Instituto Di Ricovero e Cura a Carattere Scientifico, Fondazione Don Carlo Gnocchi, Milan, 20148, Italy 6 Department of Psychology, Monash University, Melbourne, 3168, Australia 6 Department of Psychology, University of Wisconsin, Madison, WI, 53705 USA 7 Allen Institute for Brain Science, Seattle, WA, 98109 USA Abbreviated title: Is consciousness in the front versus the back of corteX? Correspondence to: Melanie Boly: [email protected] or Giulio Tononi: [email protected]. Address: 6001 Research Park Boulevard, Madison WI 53719 Number of pages: 25 Number of figures: 5 Number of words for Abstract: 45; number of words for Main teXt: 3750 Conflicts of Interest: The authors declare no conflicts of interest. Acknowledgments: This work was supported by NIH/NINDS 1R03NS096379 (to M.B.), by the Tiny Blue Dot Foundation and the Distinguished Chair in Consciousness Science (University of Wisconsin) (to G.T.), and by the James S. -

May-Britt Moser Norwegian University of Science and Technology (NTNU), Trondheim, Norway
Grid Cells, Place Cells and Memory Nobel Lecture, 7 December 2014 by May-Britt Moser Norwegian University of Science and Technology (NTNU), Trondheim, Norway. n 7 December 2014 I gave the most prestigious lecture I have given in O my life—the Nobel Prize Lecture in Medicine or Physiology. Afer lectures by my former mentor John O’Keefe and my close colleague of more than 30 years, Edvard Moser, the audience was still completely engaged, wonderful and responsive. I was so excited to walk out on the stage, and proud to present new and exciting data from our lab. Te title of my talk was: “Grid cells, place cells and memory.” Te long-term vision of my lab is to understand how higher cognitive func- tions are generated by neural activity. At frst glance, this seems like an over- ambitious goal. President Barack Obama expressed our current lack of knowl- edge about the workings of the brain when he announced the Brain Initiative last year. He said: “As humans, we can identify galaxies light years away; we can study particles smaller than an atom. But we still haven’t unlocked the mystery of the three pounds of matter that sits between our ears.” Will these mysteries remain secrets forever, or can we unlock them? What did Obama say when he was elected President? “Yes, we can!” To illustrate that the impossible is possible, I started my lecture by showing a movie with a cute mouse that struggled to bring a biscuit over an edge and home to its nest. Te biscuit was almost bigger than the mouse itself. -
Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning
The Journal of Neuroscience, August 15, 2002, 22(16):7288–7296 Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning David Gaffan,1 Alexander Easton,2 and Amanda Parker2 1Department of Experimental Psychology, Oxford University, Oxford OX1 3UD, United Kingdom, and 2School of Psychology, Nottingham University, Nottingham NG7 2RD, United Kingdom Macaque monkeys learned a strategy task in which two groups TSϩAMϩFX cannot be generally attributed to the partial tem- of visual objects needed to be treated differently, one with poral–frontal disconnection that this lesion creates, and there- persistent and one with sporadic object choices, to obtain food fore support the hypothesis that the amnesic effects of this rewards. After preoperative training, they were divided into two lesion are caused primarily by the disconnection of temporal surgical groups of three monkeys each. One group received cortex from ascending inputs from the basal forebrain. The crossed unilateral removals of frontal cortex and inferior tem- results also show that temporal–frontal interaction in strategy poral cortex (IT ϫ FC) and were severely impaired in performing implementation does not require those routes of temporal– the strategy task. The other group received bilateral transection frontal interaction that are interrupted in TSϩAMϩFX, and of anterior temporal stem, amygdala, and fornix (TSϩAMϩFX) therefore support the hypothesis that projections to other pos- and were unimpaired in performing the strategy task. Subse- terior cortical areas allow temporal and frontal cortex to interact quently the same animals were tested in visual object–reward with each other by multisynaptic corticocortical routes in strat- association learning. -

Experience Sampling During Fmri Reveals Default Network and Executive System Contributions to Mind Wandering
Experience sampling during fMRI reveals default network and executive system contributions to mind wandering Kalina Christoffa,1, Alan M. Gordonb, Jonathan Smallwoodc, Rachelle Smitha, and Jonathan W. Schoolerc aDepartment of Psychology, University of British Columbia, Vancouver, BC, Canada V6Z 2R8; bDepartment of Psychology, Stanford University, CA 94305; and cDepartment of Psychology, University of California, Santa Barbara, CA 93106 Edited by Michael I. Posner, University of Oregon, Eugene, OR, and approved March 27, 2009 (received for review February 9, 2009) Although mind wandering occupies a large proportion of our there were no online measures of mind wandering taken during waking life, its neural basis and relation to ongoing behavior scanning, it is possible that the observed default network recruit- remain controversial. We report an fMRI study that used experi- ment could be due to factors other than mind wandering. In this ence sampling to provide an online measure of mind wandering vein, Gilbert et al. (9) recently argued that instead of mind during a concurrent task. Analyses focused on the interval of time wandering, activations in the medial PFC part of the default immediately preceding experience sampling probes demonstrate network may reflect stimulus-related thought such as enhanced activation of default network regions during mind wandering, a watchfulness toward the external environment that is also likely to finding consistent with theoretical accounts of default network occur during highly practiced tasks. functions. Activation in medial prefrontal default network regions A key objective of the present study was to provide a direct was observed both in association with subjective self-reports of empirical test for the hypothesis that default network recruitment, mind wandering and an independent behavioral measure (perfor- including medial PFC regions, occurs during the precise moments mance errors on the concurrent task). -

Neuronal Properties and Synaptic Connectivity in Rodent Presubiculum Jean Simonnet
Neuronal properties and synaptic connectivity in rodent presubiculum Jean Simonnet To cite this version: Jean Simonnet. Neuronal properties and synaptic connectivity in rodent presubiculum. Neurons and Cognition [q-bio.NC]. Université Pierre et Marie Curie - Paris VI, 2014. English. NNT : 2014PA066435. tel-02295014 HAL Id: tel-02295014 https://tel.archives-ouvertes.fr/tel-02295014 Submitted on 24 Sep 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE DE DOCTORAT DE L’UNIVERSITÉ PIERRE ET MARIE CURIE Spécialité Neurosciences École doctorale Cerveau – Cognition – Comportement Présentée par : Jean Simonnet Pour obtenir le grade de DOCTEUR DE L’UNIVERSITÉ PIERRE ET MARIE CURIE Sujet de la thèse : Neuronal properties and synaptic connectivity in rodent presubiculum Soutenue le 23.09.2014 devant le jury composé de : Dr Jean-Christophe Poncer Président Dr Dominique Debanne Rapporteur Dr Maria Cecilia Angulo Rapportrice Pr Hannah Monyer Examinatrice Dr Bruno Cauli Examinateur Dr Desdemona Fricker Directrice de thèse Université Pierre & Marie Curie - Paris 6 Tél. Secrétariat : 01 42 34 68 35 Bureau d’accueil, inscription des doctorants Fax : 01 42 34 68 40 et base de données Tél. pour les étudiants de A à EL : 01 42 34 68 41 Esc. -

Encoding of 3D Head Direction Information in the Human Brain
Received: 31 May 2018 Revised: 30 October 2018 Accepted: 26 November 2018 DOI: 10.1002/hipo.23060 RESEARCH ARTICLE Encoding of 3D head direction information in the human brain Misun Kim | Eleanor A. Maguire Wellcome Centre for Human Neuroimaging, Queen Square Institute of Neurology, Abstract University College London, London, United Head direction cells are critical for navigation because they convey information about which Kingdom direction an animal is facing within an environment. To date, most studies on head direction Correspondence encoding have been conducted on a horizontal two-dimensional (2D) plane, and little is known Eleanor Maguire, Wellcome Centre for Human Neuroimaging, Queen Square Institute of about how three-dimensional (3D) direction information is encoded in the brain despite humans Neurology, University College London, and other animals living in a 3D world. Here, we investigated head direction encoding in the 12 Queen Square, London WC1N 3AR, United human brain while participants moved within a virtual 3D “spaceship” environment. Movement Kingdom. was not constrained to planes and instead participants could move along all three axes in volu- Email: [email protected] metric space as if in zero gravity. Using functional magnetic resonance imaging (fMRI) multivoxel Funding information Wellcome, Grant/Award Numbers: 101759/ pattern similarity analysis, we found evidence that the thalamus, particularly the anterior por- Z/13/Z, 102263/Z/13/Z, 203147/Z/16/Z; tion, and the subiculum encoded the horizontal component of 3D head direction (azimuth). In Samsung Scholarship contrast, the retrosplenial cortex was significantly more sensitive to the vertical direction (pitch) than to the azimuth. -

Posterior Cortex Epilepsy: Diagnostic Considerations and Surgical Outcome
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Seizure 18 (2009) 288–292 Contents lists available at ScienceDirect Seizure journal homepage: www.elsevier.com/locate/yseiz Posterior cortex epilepsy: Diagnostic considerations and surgical outcome Tao Yu, Yuping Wang, Guojun Zhang, Lixin Cai, Wei Du, Yongjie Li * Beijing Institute of Functional Neurosurgery, Xuanwu Hospital, Capital Medical University, China ARTICLE INFO SUMMARY Article history: Purpose: To assess the role of various pre-surgical evaluations in posterior cortex epilepsy (PCE) and its Received 15 January 2008 surgical outcome. Received in revised form 12 November 2008 Methods: A retrospective analysis of clinical and laboratory data from 43 PCE patients referred for Accepted 20 November 2008 surgery was performed. The diagnosis was established by standard pre-surgical evaluation modalities including semiology, MRI, interictal and ictal scalp video-EEG as well as additional intracranial EEG Keywords: monitoring in selected cases. Posterior cortex Results: The 43 patients included 11 parietal lobe epilepsies, 13 occipital lobe epilepsies, and 19 patients Epilepsy surgery with seizures originating from parieto-occipito-posterior temporal cortex. Thirty-three (76.7%) patients Surgical outcome experienced at least one type of aura. Seventeen patients showed complex focal seizures, which were followed by secondarily generalized tonic–clonic seizures in seven of them; eighteen patients predominantly showed simple motor seizures (clonic seizures in 6, tonic seizures in 7, and versive seizures in 5). Long-term visual field deficits were observed in 8 patients. Other transient neurological deficits occurred in 7 patients. All patients received the follow-up study lasting 1–5 years, and achieved Engel’s I in 26 cases, II in 5, III in 5, and IV–V in 7.